- Dextropropoxyphene
-
Propoxyphene 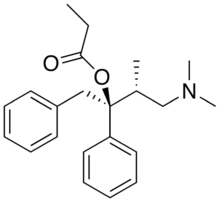
Systematic (IUPAC) name (1S,2R)-1-benzyl-3-(dimethylamino)-2-methyl-1-phenylpropyl propionate Clinical data Trade names Darvon AHFS/Drugs.com monograph MedlinePlus a682325 Licence data US FDA:link Pregnancy cat. C, D for prolonged use (US) Legal status Prescription Only (S4) (AU) Schedule II, Schedule IV (in dosage form) (US) Routes oral, IV, rectal Pharmacokinetic data Half-life 3.6–6.5 hours[1] Identifiers CAS number 469-62-5 
ATC code N02AC04 PubChem CID 10100 DrugBank DB00647 ChemSpider 9696 
UNII S2F83W92TK 
KEGG D07809 
ChEBI CHEBI:51173 
ChEMBL CHEMBL1213351 
Chemical data Formula C22H29NO2 Mol. mass 339.471 g/mol SMILES eMolecules & PubChem Physical data Melt. point 75 °C (167 °F)  (what is this?) (verify)
(what is this?) (verify)Dextropropoxyphene[2], manufactured by Eli Lilly and Company, is an analgesic in the opioid category. It is intended to treat mild pain and has, in addition, anti-tussive and local anesthetic effects. It has been taken off the market in Europe and the US due to concerns of fatal overdoses and arrhythmias.[3] An estimated 10 million patients have used these products.
Dextropropoxyphene is sometimes combined with paracetamol or acetylsalicylic acid. Trade-names include Darvocet-N and Di-Gesic [4] Darvon with APAP for dextropropoxyphene and paracetamol and Darvon with ASA for dextropropoxyphene and aspirin.[5] The British Approved Name (i.e. the generic name of the active ingredient) of the paracetamol/dextropropoxyphene preparation is co-proxamol (sold under a variety of brand names); however, it has been withdrawn since 2007, and is no longer available to new patients.[6] The paracetamol combination(s) are known as Capadex or Di-Gesic in Australia, Lentogesic in South Africa, and Di-Antalvic in France (unlike co-proxamol, which is an approved name, these are all brand names).
Contents
Uses
Analgesia
Dextropropoxyphene, like codeine, is a weak opioid, known to cause dependency among recreational users. Codeine is more commonly used; however, as codeine is, in essence, a prodrug that requires in vivo metabolism to the more active opioid morphine for maximum efficacy, it is ineffective for some individuals with the "poor metabolizer" genotype of the liver cytochrome P450 enzyme CYP2D6[citation needed]. It is in people with this low-function isoform of the CYP2D6 gene that dextropropoxyphene is particularly useful, as its metabolism does not require CYP2D6. It is also used for patients with digestive complaints as it is less liable to worsen their symptoms.
Restless legs syndrome (RLS)
Dextropropoxyphene has been found to be helpful in relieving the symptoms of restless legs syndrome (RLS).[7][8][9]
Opioid withdrawal
In pure form, dextropropoxyphene is commonly used to ease the withdrawal symptoms in people addicted to opioids. Being very weak in comparison to the opioids that are commonly abused, dextropropoxyphene can only act as a "partial" substitute. It does not have much effect on mental cravings; however it can be effective in alleviating physical withdrawal effects, such as muscle cramps.
Contraindications
Dextropropoxyphene is contraindicated in patients allergic to paracetamol or dextropropoxyphene, in alcoholics; and in combination with amphetamine. Dextropropoxyphene is not intended for use in patients who are prone to suicide or addiction.
Side effects
- Constipation
- Itching
- Drowsiness
- Sore throat
- Impaired alertness
- Confusion
- Serious or fatal heart rhythms




Pharmacology
Dextropropoxyphene acts as a mu-opioid receptor agonist. It also acts as a potent, noncompetitive α3β4 neuronal nicotinic acetylcholine receptor antagonist,[10] as well as weak serotonin reuptake inhibitor.
Toxicity
Overdose is commonly broken into two categories: liver toxicity (from paracetamol poisoning) and dextropropoxyphene overdose.
Many users experience toxic effects from the paracetamol (acetaminophen) in pursuit of the endlessly-increasing dose required for pain relief. They suffer acute liver toxicity, which causes severe stomach pains, nausea, and vomiting (all of which are increased by light or stimulation of the sense of sight).
An overdose of dextropropoxyphene may lead to various systemic effects. Excessive opioid receptor stimulation is responsible for the CNS depression, respiratory depression, miosis, and gastrointestinal effects seen in propoxyphene poisoning. It may also account for mood/thought altering effects.
In addition, both propoxyphene and its metabolite norpropoxyphene have local anesthetic effects at concentrations about 10 times those necessary for opioid effects. Norpropoxyphene is a more potent local anesthetic than propoxyphene, and they are both more potent than lidocaine.[11] Local anesthetic activity appears to be responsible for the arrhythmias and cardiovascular depression seen in propoxyphene poisoning.[12]
Both propoxyphene and norpropoxyphene are potent blockers of cardiac membrane sodium channels and are more potent than lidocaine, quinidine, and procainamide in this respect.[13] As a result, propoxyphene and norpropoxyphene appear to have the characteristics of a Vaughn-Williams Class Ic antiarrhythmic.
These direct cardiac effects include decreased heart rate (i.e. cardiovascular depression), decreased contractility, and decreased electrical conductivity (i.e., increased PR, AH, HV, and QRS intervals). These effects appear to be due to their local anesthetic activity and are not reversed by naloxone.[11][12][14] Widening of the QRS complex appears to be a result of a quinidine-like effect of propoxyphene, and sodium bicarbonate therapy appears to have a positive direct effect on the QRS dysrhythmia.[15]
Seizures may result from either opioid or local anesthetic effects.[11] Pulmonary edema may result from direct pulmonary toxicity, neurogenic/anoxic effects, or cardiovascular depression.[12]
Available forms
Propoxyphene was initially introduced as propoxyphene hydrochloride. Shortly before the patent on propoxyphene expired, propoxyphene napsylate form was introduced to the market. Napsylate salt is claimed to be less prone to abuse, because it is almost insoluble in water and therefore cannot be used for injection. Napsylate also gives lower peak blood level.[16] Because of different molecular mass, a dose of 100 mg of propoxyphene napsylate is required to supply an amount of propoxyphene equivalent to that present in 65 mg propoxyphene hydrochloride.
Before the FDA-directed recall, dextropropoxyphene HCl was available in the United States as a prescription formulation with paracetamol (acetaminophen) in ratio anywhere from 30 mg / 600 mg to 100 mg / 650 mg (or 100 mg / 325 mg in the case of Balacet), respectively. These are usually named "Darvocet." On the other hand, "Darvon" is a pure propoxyphene preparation that does not contain paracetamol.
In Australia, dextropropoxyphene is available on prescription, both as a combined product (32.5 mg dextropropoxyphene per 325 mg paracetamol branded as either "Di-gesic", "Capadex", and "Paradex," it is also available in pure form (100 mg capsules) known as "Doloxene".
Drug testing
Detectable levels of propoxyphene/dextropropoxyphene may stay in a person's system for up to nine days after last dose and can be tested for specifically in non-standard urinalysis but may remain in the body longer in tiny amounts. Propoxyphene will not show up on standard opiate/opioid tests because it is not chemically related to opiates part of the OPI or OPI 2000 panels, which detect morphine and related compounds. It is most closely related to Methadone.
Usage controversy and regulation
Dextropropoxyphene is subject to some controversy: while many physicians prescribe it for a wide range of mildly to moderately painful symptoms as well as for treatment of diarrhea, many others refuse to prescribe it, citing limited effectiveness.[citation needed] In addition, the therapeutic index of dextroproxyphene is relatively small.
Caution should be used when administering dextropropoxyphene, particularly with children and the elderly and with patients who may be pregnant or breast feeding[citation needed]; other reported problems include kidney, liver or respiratory disorders, and prolonged use. Attention should be paid to concomitant use with tranquilizers, antidepressants or excess alcohol.
Darvon, a dextropropoxyphene made by Eli Lilly, which had been on the market for 25 years, came under heavy fire in 1978 by consumer groups that said it was associated with suicide.[citation needed] Darvon was never withdrawn from the market, but Lilly has waged a sweeping, and largely successful, campaign[citation needed] among doctors, pharmacists and Darvon users to defend the drug as safe when it is used in proper doses and not mixed with alcohol. On November 19, 2010, the FDA banned all sale of Darvon and Darvocet.[17]
Australia
In Australia, both pure dextropropoxyphene capsules (as napsylate, 100 mg), marketed as "Doloxene", and combination tablets and capsules (with paracetamol) all containing 32.5 mg dextropropoxyphene HCl with 325 mg paracetamol, continue to remain available by prescription.[18]
European Union
In November 2007, the European Commission requested the European Medicines Agency (EMEA) to review the safety and effectiveness of dextropropoxyphene based medicines and on 25 June 2009 the EMEA recommended a gradual withdrawal throughout the European Union. The EMEA's conclusion was based on evidence that dextropropoxyphene-containing medicines were weak painkillers, the combination of dextropropoxyphene and paracetamol was no more effective than paracetamol on its own, and that the difference between the dose needed for treatment and a harmful dose (the "therapeutic index") was too small.[19]
New Zealand
In February 2010, Medsafe announced that Paradex and Capadex (forms of dextropropoxyphene) were being withdrawn from the marketplace due to health issues, and withdrawal in other countries.[20]
Sweden
In Sweden physicians have earlier been discouraged by the medical products agency to prescribe dextropropoxyphene due to the risk of respiratory depression when taken with alcohol.[21] Products with mixed active ingredients where taken off the market and only products with dextropropoxyphene where earlier supposed to be sold. Physicians have eariler been recommended to prescribe products with only dextropropoxyphene and not to patients with a history of drug abuse, depression or suicidal tendencies.
- As of march 2011 all products containing the substance are withdrawn because of safety issues after EU directive.′′[22]
- It was discussed at the time that people who abuse alcohol and other substances and take combination dextropoxyphene/acetaminophen (paracetamol) may need to take many combination tablets to reach euphoria. This is because the amount of dextropropoxyphene per tablet is relatively low (30–40 mg). The ingested paracetamol - the other component - may then reach liver toxic levels. In the case of alcoholics, who often already have damaged livers, even a relatively small overdose with paracetamol may produce hepatotoxicity . The toxicity of the combination of overdosed dextropoxyphene with its CNS/respiratory depression and paracetamol induced liver damage can be a recipe for disaster or death. This is also true for any person with an already damaged or weakened liver by disease or otherwise.
United Kingdom
In the United Kingdom, preparations containing only dextropropoxyphene were discontinued in 2004.[23] In 2007, the Medicines and Healthcare products Regulatory Agency (MHRA) removed the licence for co-proxamol. From then onwards, in the UK co-proxamol is only available on a named patient basis, for long term chronic pain and only to those who have already been prescribed this medicine. Its withdrawal from the UK market is a result of concerns relating to its toxicity in overdose (even small overdose can be fatal), and dangerous reaction with alcohol. Recreational use in the UK is uncommon. Many patients have been prescribed alternative combinations of drugs as a replacement.[24]
The MHRA's motivation for the withdrawal of co-proxamol was the reduction in suicides and a key part of its justification of its decision was based upon studies showing that co-proxamol was no more effective than paracetamol alone in pain management.[25] Prescribing authorities such as the Royal College of General Practitioners unanimously recommended withdrawal, while patients who responded to the MHRA's request for information tended to want to continue treatment.[26] Many doctors as well as patients believe that clinical experience shows co-proxamol is more effective than paracetamol alone.[citation needed]
The co-proxamol preparations available in the UK contained a sub-therapeutic dose of paracetamol, 325 mg per tablet.[27] Patients were warned not to take more than eight tablets in one day, a total dose of 2600 mg paracetamol per day. This is in comparison to the 4000 mg daily limit on paracetamol alone, a significantly higher dose. Despite this reduced level, patients were still at a high risk of overdose: coproxamol was second only to tricyclic antidepressants as the most common prescription drugs used in overdose.[25] Following the reduction in prescribing in 2005–2007, prior to its complete withdrawal, the number of deaths associated with the drug dropped significantly. Additionally, patients have not substituted other drugs as a method of overdose.[28]
The decision to withdraw coproxamol has met with some controversy; it has been brought up in the House of Commons on two occasions, 13 July 2005[29] and on 17 January 2007.[30] Patients have found alternatives to co-proxamol either too strong, too weak, or with intolerable side effects.[citation needed] During the House of Commons debates, it is quoted that originally some 1,700,000 patients in the UK were prescribed co-proxamol. Following the MHRA phased withdrawal this has eventually been reduced to 70,000. However, it appears this is the residual pool of patients who cannot find alternate analgesia to co-proxamol.[citation needed]
The MHRA safety net of prescribing co-proxamol after licence withdrawal from 31 December 2007, on a "Named Patient" basis where doctors agree there is a clinical need, has been rejected by most UK doctors[citation needed] because the MHRA wording that "responsibility will fall on the prescriber" is unacceptable to most doctors. Some patients intend to take the case to the European Court of Human Rights.[31] However, the European Medicines Agency has recently backed the MHRA's decision, and recommended in June 2009 that propoxyphene preparations be withdrawn across the European Union.[32]
United States
In January 2009, an FDA advisory committee voted 14 to 12 against the continued marketing of propoxyphene products, based on its weak pain-killing abilities, addictiveness, association with drug deaths and possible heart problems, including arrhythmia. A subsequent re-evaluation resulted in a July 2009 recommendation to strengthen the boxed warning for propoxyphene to reflect the risk of overdose.[33] Dextropropoxyphene currently carries a black box warning in the U.S., stating:
Propoxyphene should be used with extreme caution, if at all, in patients who have a history of substance/drug/alcohol abuse, depression with suicidal tendency, or who already take medications that cause drowsiness (e.g., antidepressants, muscle relaxants, pain relievers, sedatives, tranquilizers). Fatalities have occurred in such patients when propoxyphene was misused.[34]
Because of potential for side effects, this drug is on the list for High Risk Medications in the elderly.[35]
On November 19, 2010, the FDA requested the cessation of all sale of Darvon and Darvocet from the US drug market due to heart arrhythmia in patients who took the drug.[36] The drug Darvocet may also be involved in combined drug intoxication because it may lead to confusion in patients and physicians. Many doctors are commonly switching to tramadol, because it is generally considered safer.[citation needed]
Canada
On December 1, 2010, Health Canada and Paladin Labs Inc. announced the voluntary recall and withdrawal of Darvon-N from the Canadian market and the discontinuation of sale of Darvon-N.[37]
Use by right to die societies
High toxicity and relatively easy availability made propoxyphene drug of choice for right to die societies. Propoxyphene is listed in Dr. Philip Nitschke's The Peaceful Pill Handbook and Dr. Pieter Admiraal's Guide to a Human Self-Chosen Death.[38][39] "With the withdrawal of the barbiturate sleeping tablets from the medical prescribing list, propoxyphene has become the most common doctor-prescribed medication used by seriously ill people to end their lives."[38] The slang name for the combination of propoxyphene and other drugs used for suicide is "Darvon cocktail".[40]
References
- ^ Slywka GW, Melikian AP, Whyatt PL, Meyer MC (1975). = long&pmid = 1150913 "Propoxyphene bioavailability: an evaluation of ten products". J Clin Pharmacol 15 (8-9): 598–604. PMID 1150913. http://jcp.sagepub.com/cgi/pmidlookup?view = long&pmid = 1150913. Retrieved 2008-06-16.
- ^ US Patent 2728779 - Esters of Substituted Aminobutanes
- ^ Physicians Say Good Riddance to 'Worst Drug in History' By Allison Gandey. February 2, 2011
- ^ "Consumer Medicine Information: Digesic". Aspen Pharmacare Australia Pty Ltd. http://www.aspenpharma.com.au/PDF/CMI/CMI_Digesic.pdf.
- ^ Nursing Drug Handbook, Springhouse, page 306
- ^ BNF Edition 57, BNF.org
- ^ "Restless legs syndrome: Definition from". Answers.com. http://www.answers.com/topic/restless-legs-syndrome. Retrieved 2009-08-19.
- ^ "Restless Leg Syndrome - Sleep Medicine Centers of WNY". Sleepmedicinecenters.com. http://www.sleepmedicinecenters.com/Home/SleepDisorders/RestlessLegsSyndrome. Retrieved 2009-08-19.
- ^ "Causes, diagnosis and treatment for the patient living with Restless Legs Syndrome (RLS)". Restless Leg Syndrome Foundation. 1 April 2006. http://www.rls.org/Document.Doc?&id=3. Retrieved 2009-08-19.
- ^ "Blockade of Rat α3β4 Nicotinic Receptor Function by Methadone, Its Metabolites, and Structural Analogs — JPET". http://jpet.aspetjournals.org/content/299/1/366.abstract.
- ^ a b c Nickander et al., 1984
- ^ a b c Strom et al., 1985b
- ^ Holland & Steinberg, 1979
- ^ Bredgaard, Sorensen et al., 1984
- ^ Stork et al., 1995
- ^ Wilson, Charles Owens and Gisvold, John H. Wilson and Gisvold's textbook of organic medicinal and pharmaceutical chemistry. Lippincott Williams & Wilkins. ISBN 0781734819.
- ^ "FDA pulls common pain med off the market". November 19, 2010. CNN.
- ^ MIMS No. 2 2008 Section 4a
- ^ "Questions and answers on the withdrawal of the marketing authorisations for medicines containing dextropropoxyphene". European Medicines Agency. 25 June 2009. http://www.emea.europa.eu/pdfs/human/referral/dextropropoxyphene/40106109en.pdf. Retrieved 2009-09-08.
- ^ "Paradex And Capadex To Be Withdrawn From NZ". http://www.scoop.co.nz/stories/GE1002/S00034.htm. Retrieved 2010-02-21.
- ^ "Fixed combinations of analgesic drugs containing to be removed from the market in the autumn of 2005" (in swedish). 5 May 2005. http://www.lakemedelsverket.se/Tpl/NewsPage____495.aspx.
- ^ "Dextropropoxyphene is removed from the market" (in swedish). 5 May 2005. http://www.lakemedelsverket.se/Alla-nyheter/NYHETER-2010/Dextropropoxifen-dras-bort-fran-marknaden/.
- ^ Painkiller Scrapped Over Suicides
- ^ BNF.org, BNF edition 57, Retrieved 28 August 2009
- ^ a b "Withdrawal of co-proxamol products and interim updated prescribing information". Medicines and Healthcare products Regulatory Agency (MHRA). 31 January 2005. Retrieved August 28, 2009.
- ^ "Outcome of the public request for information on the risks and benefits of the pain killer co-proxamol". Medicines and Healthcare products Regulatory Agency (MHRA).
- ^ "Co-proxamol: outcome of the review of risks and benefits". Questions and Answers leaflet, Retrieved August 28, 2009
- ^ Hawton K, Bergen H, Simkin S, Brock A, Griffiths C, Romeri E, Smith KL, Kapur N, Gunnell D (June 2009). "Effect of withdrawal of co-proxamol on prescribing and deaths from drug poisoning in England and Wales: time series analysis". BMJ 338: b2270. doi:10.1136/bmj.b2270. PMID 19541707. http://www.bmj.com/content/338/bmj.b2270.full.pdf.
- ^ Co-Proxamol: 13 Jul 2005: House of Commons debates (TheyWorkForYou.com)
- ^ Co-proxamol: 17 Jan 2007: Westminster Hall debates (TheyWorkForYou.com)
- ^ Failure Of MHRA Coproxamol Named Patient System - Visitor Opinion
- ^ News Centre : MHRA
- ^ "FDA Takes Actions on Darvon, Other Pain Medications Containing Propoxyphene". U.S. Food and Drug Administration (FDA). 7 July 2009. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170769.htm.
- ^ Drug Information for Darvocet-N 100 Oral - Web MD
- ^ NCQA's HEDIS Measure: Use of High Risk Medications in the Elderly
- ^ Zajac, Andrew (November 19, 2010). "Darvon, Darvocet painkillers pulled from the U.S. market". L.A. Times. http://www.latimes.com/news/nationworld/nation/sc-dc-1120-fda-darvon-web-20101119,0,6525838.story. Retrieved November 19, 2010.[dead link]
- ^ Health Canada: Darvon-N (dextropopoxyphene) - Recall and Withdrawal in Canada
- ^ a b Nitschke, Philip & Fiona Stewart (2006). The Peaceful Pill Handbook. U.S.: Exit International. ISBN 0978878817.
- ^ Dr. Pieter Admiraal et al.. Guide to a Humane Self-Chosen Death. The Netherlands: WOZZ Foundation, Delft. ISBN 9078581018.
- ^ ASH Wiki: Darvon Cocktail
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersCategories:- Synthetic opioids
- Amines
- Antitussives
- Combination drugs
- Eli Lilly and Company
- Mu-opioid agonists
- Propionates
Wikimedia Foundation. 2010.
Look at other dictionaries:
Dextropropoxyphene — Dextropropoxyphène Pour les articles homonymes, voir DPX. Dextropropoxyphène … Wikipédia en Français
dextropropoxyphène — ● dextropropoxyphène nom masculin Analgésique de synthèse possédant une action voisine des salicylés, mais d efficacité nettement supérieure. (Son action sédative est moins intense que celle de la morphine. Il est peu toxicomanogène.) … Encyclopédie Universelle
Dextropropoxyphène — Pour les articles homonymes, voir DPX. Dextropropoxyphène Général … Wikipédia en Français
dextropropoxyphene — noun A particular narcotic painkiller … Wiktionary
dextropropoxyphene — dex·tro·pro·poxy·phene .dek strə prō päk sə .fēn n PROPOXYPHENE * * * n. an analgesic used to relieve mild or moderate pain. It is administered by mouth, often in combination with other analgesics (see co proxamol), and sometimes causes dizziness … Medical dictionary
dextropropoxyphene — dex·tro·propoxyphene … English syllables
dextropropoxyphene — ¦dekstrəˌ noun Etymology: dextr + propoxyphene (herein) : propoxyphene herein … Useful english dictionary
dextropropoxyphene hydrochloride — SYN: propoxyphene hydrochloride … Medical dictionary
dextropropoxyphene napsylate — SYN: propoxyphene napsylate … Medical dictionary
469-62-5 — Dextropropoxyphène Pour les articles homonymes, voir DPX. Dextropropoxyphène … Wikipédia en Français

