- Malathion
-
Malathion 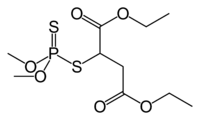
 Diethyl 2-[(dimethoxyphosphorothioyl)sulfanyl]butanedioateOther names2-(dimethoxyphosphinothioylthio) butanedioic acid diethyl ester
Diethyl 2-[(dimethoxyphosphorothioyl)sulfanyl]butanedioateOther names2-(dimethoxyphosphinothioylthio) butanedioic acid diethyl ester
Malathion
Carbofos
Maldison
Mercaptothion
Ortho malathionIdentifiers CAS number 121-75-5 
PubChem 4004 ChemSpider 3864 
UNII U5N7SU872W 
DrugBank DB00772 KEGG D00534 
ChEBI CHEBI:6651 
ChEMBL CHEMBL1200468 
ATC code P03,QP53AF12 Jmol-3D images Image 1 - O=C(OCC)C(SP(=S)(OC)OC)CC(=O)OCC
Properties Molecular formula C10H19O6PS2 Molar mass 330.358021 Appearance Clear colorless liquid Density 1.23 g/cm3 Melting point 2.9 °C
Boiling point 156-157 °C at 0.7 mmHg
Solubility in water 145 mg/L at 20 °C[1] Solubility Soluble in ethanol and acetone; very soluble in ethyl ether log P 2.36 (octanol/water)[2]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references "Ovide" redirects here. For the animated series Ovide and the Gang, see Ovide and the Gang.Malathion is an organophosphate parasympathomimetic which binds irreversibly to cholinesterase. Malathion is an insecticide of relatively low human toxicity, however one recent study has shown that children with higher levels of organophosphate pesticide metabolites in their urine are more likely to have attention deficit hyperactivity disorder.[3]
In the former USSR it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion.[4]
Contents
Pesticide use
Malathion is a pesticide that is widely used in agriculture, residential landscaping, public recreation areas, and in public health pest control programs such as mosquito eradication.[5] In the US, it is the most commonly used organophosphate insecticide.[6]
Malathion was used in the 1980s in California to combat the Mediterranean Fruit Fly. This was accomplished on a wide scale by the near weekly aerial spraying of suburban communities for a period of several months. Formations of three or four agricultural helicopters would overfly suburban portions of Alameda County, San Bernardino County, San Mateo County, Santa Clara County, San Joaquin County, Stanislaus County, and Merced County releasing a mixture of malathion and corn syrup, the corn syrup being a bait for the fruit flies. Malathion has also been used to combat the Mediterranean fruit fly in Australia.[7]
Malathion was sprayed in many cities to combat West Nile virus. In the Fall of 1999 and the Spring of 2000, Long Island and the five boroughs of New York City were sprayed with several pesticides, one of which was malathion. While it was claimed by some anti-pesticide groups that use of these pesticides caused a lobster die-off in Long Island Sound, there is as of yet no conclusive evidence to support this.[8]
Manitoba ordered the city of Winnipeg, Manitoba to be sprayed in July 2005 as part of the West Nile virus campaign. Prior to this, malathion was used over the last couple of decades on a regular basis during summer months to kill nuisance mosquitoes, but homeowners were allowed to exempt their properties if they chose. Today, Winnipeg is the only major city in Canada with an ongoing Malathion nuisance-adult-mosquito-control program.
Malathion is also used in conjunction with diesel fuel to fog an area where there is an infestation of mosquitoes. By diluting the mixture, it becomes much weaker. It is possible to dilute the mixture to the point where mosquitoes are not killed, but become more resistant to the mixture, making it less effective in subsequent foggings.
Medical use
Malathion in low doses (0.5% preparations) is used as a treatment for:
- head lice and body lice. Malathion and lindane are the only two agents approved by the FDA for treatment of pediculosis.[9] It is claimed to effectively kill both the eggs and the adult lice, but in fact has been shown in UK studies to be only 36% effective on head lice, and less so on their eggs.[10] This low efficiency was found when malathion was applied to lice found on schoolchildren in the Bristol area in the UK and it is assumed to be caused by the lice having developed resistance against malathion.
Preparations include Derbac-M, Prioderm, Quellada-M[12] and Ovide.[13]
Risks
General
Malathion itself is of low toxicity; however, absorption or ingestion into the human body readily results in its metabolism to malaoxon, which is substantially more toxic.[14] Chronic exposure to low levels of malathion have been hypothesized to impair memory, but this is disputed. According to the United States Environmental Protection Agency there is currently no reliable information on adverse health effects of chronic exposure to malathion.[15] Acute exposure to extremely high levels of malathion will cause body-wide symptoms whose intensity will be dependent on the severity of exposure. Possible symptoms include skin and eye irritation, cramps, nausea, diarrhea, excessive sweating, seizures and even death. Most symptoms tend to resolve within several weeks. Malathion present in untreated water is converted to malaoxon during the chlorination phase of water treatment, so malathion should not be used in waters that may be used as a source for drinking water, or any upstream waters.
In 1981, B. T. Collins, Director of the California Conservation Corps, publicly swallowed and survived a mouthful of dilute Malathion solution. This was an attempt to demonstrate Malathion's safety following an outbreak of Mediterranean fruit flies in California. Malathion was sprayed over a 1,400 sq mi (3,600 km2). area to control the flies.[16]
In 1976, numerous malaria workers in Pakistan were poisoned by isomalathion, a contaminant that may be present in some preparations of malathion.[17] It is capable of inhibiting carboxyesterase enzymes in those exposed to it. It was discovered that poor work practices had resulted in excessive direct skin contact with isomalathion contained in the malathion solutions. Implementation of good work practices, and the cessation of use of malathion contaminated with isomalathion led to the cessation of poisoning cases.
Malathion breaks down into Malaoxon. In studies of the effects of long-term exposure to oral ingestion of malaoxon in rats, malaoxon has been shown to be 61 times more toxic than malathion.[14]
If malathion is used in an indoor, or other poorly ventilated environment, it can seriously poison the occupants living or working in this environment. A possible concern is that malathion being used in an outdoor environment, could enter a house or other building; however, studies by the EPA have conservatively estimated that possible exposure by this route is well below the toxic dose of malathion.[14] Regardless of this fact, in jurisdictions which spray malathion for pest control, it is often recommended to keep windows closed and air conditioners turned off while spraying is taking place, in an attempt to minimize entry of malathion into the closed environment of residential homes.
Although current EPA regulations do not require amphibian testing, a 2008 study done by the University of Pittsburgh found that "cocktails of contaminants", which are frequently found in nature, were lethal to leopard frog tadpoles. They found that a combination of five widely used insecticides (carbaryl, chlorpyrifos, diazinon, endosulfan, and malathion) in concentrations far below the limits set by the EPA killed 99% of leopard frog tadpoles.[18]
A May 2010 study found that in a representative sample of US children, those with higher levels of organophosphate pesticide metabolites in their urine were more likely to have attention-deficit/hyperactivity disorder. Each 10-fold increase in urinary concentration of organophosphate metabolites was associated with a 55% to 72% increase in the odds of ADHD. The study was the first investigation on children's neurodevelopment to be conducted in a group with no particular pesticide exposure[19][20]
Carcinogenicity
Malathion is classified by US EPA as having “suggestive evidence of carcinogenicity but not sufficient to assess human carcinogenic potential.”[21] This rating implies that insufficient evidence is available to either rule out malathion as a carcinogen, or to state that it is a carcinogen. No studies on carcinogenicity have been performed in humans; however, studies in rats and mice have yielded conflicting results. Liver tumours were found to be induced in rats, but only at excessive doses. On the other hand, malaoxon, a structurally related chemical, was found not to induce tumour formation in rats. A review of the classification of malathion as 'suggestive' was carried out in 2000, by the FIFRA Scientific Advisory Panel. The conclusion of this panel was that there was still insufficient evidence to either declare malathion as non-carcinogenic, or to declare it a carcinogen.
See also
References
- ^ Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium, 11 th ed., British Crop Protection Council, Surrey, England 1997, p. 755
- ^ Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society., 1995., p. 80
- ^ Maugh II, Thomas H. (16 May 2010). "Study links pesticide to ADHD in children". Los Angeles Times. http://articles.latimes.com/2010/may/16/science/la-sci-pesticides-20100517.
- ^ "alanwood.net". http://www.alanwood.net/pesticides/malathion.html. Retrieved 2007-09-16.
- ^ Malathion for mosquito control, US EPA
- ^ Bonner MR, Coble J, Blair A et al. (2007). "Malathion Exposure and the Incidence of Cancer in the Agricultural Health Study". American Journal of Epidemiology 166 (9): 1023–1034. doi:10.1093/aje/kwm182. PMID 17720683.
- ^ Edwards JW, Lee SG, Heath LM, Pisaniello DL (2007). "Worker exposure and a risk assessment of malathion and fenthion used in the control of Mediterranean fruit fly in South Australia". Environ. Res. 103 (1): 38–45. doi:10.1016/j.envres.2006.06.001. PMID 16914134.
- ^ ModelContaminants_Toxic Effec
- ^ Amy J. McMichael; Maria K. Hordinsky (2008). Hair and Scalp Diseases: Medical, Surgical, and Cosmetic Treatments. Informa Health Care. pp. 289–. ISBN 9781574448221. http://books.google.com/?id=W-dT_21KZOIC&pg=PA289. Retrieved 27 April 2010.
- ^ Downs AM, Stafford KA, Harvey I, Coles GC (1999). "Evidence for double resistance to permethrin and malathion in head lice". Br. J. Dermatol. 141 (3): 508–11. doi:10.1046/j.1365-2133.1999.03046.x. PMID 10583056.
- ^ Julia A. McMillan; Ralph D. Feigin; Catherine DeAngelis; M. Douglas Jones (1 April 2006). Oski's pediatrics: principles & practice. Lippincott Williams & Wilkins. pp. 1–. ISBN 9780781738941. http://books.google.com/?id=VbjFQiz8aR0C&pg=RA1-PA1383. Retrieved 27 April 2010.
- ^ British National Formulary 54th Ed. Sept 2007. ISBN 9780853697367. ISSN0260-535X
- ^ "AHFS Drug Information". American Society of Health-System Pharmacists. 13 January 2011. http://www.medicinescomplete.com/mc/ahfs/current/a384040.htm. Retrieved 17 January 2011.
- ^ a b c Edwards D (2006). "Reregistration Eligibility Decision for Malathion". US Environmental Protection Agency - Prevention, Pesticides and Toxic Substances EPA 738-R-06-030 journal: 9. http://www.epa.gov/pesticides/reregistration/REDs/malathion_red.pdf.
- ^ "US Department of Health and Human Services: Agency for Toxic Substances and Disease Registry - Medical Management Guidelines for Malathion". http://www.atsdr.cdc.gov/MHMI/mmg154.html. Retrieved 2008-04-02.
- ^ Bonfante, Jordan (1990-01-08). "Medfly Madness". TIME. http://www.time.com/time/magazine/article/0,9171,969129,00.html. Retrieved May 21, 2009.
- ^ Baker EL, Warren M, Zack M et al. (1978). "Epidemic malathion poisoning in Pakistan malaria workers". Lancet 1 (8054): 31–4. PMID 74508.
- ^ http://www.sciencedaily.com/releases/2008/11/081111183041.htm
- ^ http://www.medscape.com/viewarticle/721892
- ^ Bouchard, Maryse F.; Bellinger, David C.; Wright, Robert O.; Weisskopf, Marc G. (2010-05-17), "Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides" (PDF), Pediatrics 125 (6): e1270-e1277, doi:10.1542/peds.2009-3058, ISSN 0031-4005, http://pediatrics.aappublications.org/cgi/reprint/peds.2009-3058v1.pdf, retrieved 2010-08-23. Online ISSN 1098-4275. Alternate URL [1]. HTML version without inline tables [2].
- ^ Reregistration Eligibility Decision for Malathion, US EPA
External links
- Malathion Technical Fact Sheet - National Pesticide Information Center
- Malathion General Fact Sheet - National Pesticide Information Center
- Malathion Pesticide Information Profile - Extension Toxicology Network
- ATSDR ToxFAQs
- Re-evaluation of Malathion by the Pest Management Regulatory Agency of Canada
Ectoparasiticides / arthropod (P03A) Insecticide/pediculicide Chlorine-containing productsMalathionAcaricide/miticide/scabicide PyrethrinesSulfur-containing productsChlorine-containing productsMalathionOther/ungroupedM: IFT
helm,arth (acar)
helm, arth (lice), zoon
helm, arth
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Insecticides
- Antiparasitic agents
- Endocrine disruptors
- Organophosphate insecticides
- Anticholinesterases
- Phosphorodithioates
Wikimedia Foundation. 2010.
Look at other dictionaries:
Malathion — Général No CAS … Wikipédia en Français
Malathion — Malathion. См. малатион. (Источник: «Англо русский толковый словарь генетических терминов». Арефьев В.А., Лисовенко Л.А., Москва: Изд во ВНИРО, 1995 г.) … Молекулярная биология и генетика. Толковый словарь.
malathion — ☆ malathion [mal΄ə thī′än΄, mal΄ə thī′ən ] n. [< MAL(IC) A(CID) + THION(IC)] an organic phosphate, C10H19O6S2P, of relatively low toxicity for mammals, used as an insecticide … English World dictionary
Malathion® — /ma lə thīˈon/ noun A phosphorus containing insecticide used chiefly in the house and garden ORIGIN: From diethyl maleate and ↑thio … Useful english dictionary
Malathion — Strukturformel Allgemeines Name Malathion Andere Namen … Deutsch Wikipedia
malathion — /mal euh thuy on, euhn/, n. an organic phosphate insecticide, C10H19O6S2P, of relatively low toxicity for mammals. [1953] * * * ▪ insecticide trade name for an organic phosphorus compound that is a general purpose insecticide considerably… … Universalium
malathion — noun Etymology: from Malathion, a trademark Date: 1953 an organophosphate broad spectrum insecticide C10H19O6PS2 that is considerably less toxic to mammals than parathion … New Collegiate Dictionary
malathion — Organofosfato empleado para eliminar una serie de plagas de insectos en agricultura, salud pública y jardinería. Aunque no se considera de forma general muy tóxico para los seres humanos, las personas sensibles a los organofosfatos deberían… … Diccionario ecologico
malathion — karbofosas statusas T sritis chemija apibrėžtis Insekticidas. formulė (CH₃O)₂P(=S)SCH(COOC₂H₅)CH₂COOC₂H₅ atitikmenys: angl. carbophos; malathion rus. карбофос ryšiai: sinonimas – dietil [(dimetoksifosfinotiol)tio]butandioatas … Chemijos terminų aiškinamasis žodynas
malathion poisoning — noun a toxic condition caused by inhaling or ingesting the insecticide Malathion • Hypernyms: ↑pesticide poisoning … Useful english dictionary
