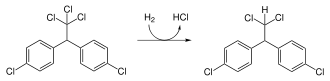
- Dichlorodiphenyldichloroethane
-
Dichlorodiphenyldichloroethane 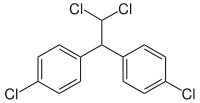 1-chloro-4-[2,2-dichloro-1-(4-
1-chloro-4-[2,2-dichloro-1-(4-
chlorophenyl)ethyl]benzeneIdentifiers Abbreviations DDD CAS number 72-54-8 
PubChem 6294 ChemSpider 6057 
EC number 200-783-0 KEGG C06636 
MeSH DDD ChEBI CHEBI:27841 
ChEMBL CHEMBL196590 
Beilstein Reference 4-05-00-01884 Jmol-3D images Image 1 - Clc1ccc(cc1)C(c2ccc(Cl)cc2)C(Cl)Cl
Properties Molecular formula C14H10Cl4 Molar mass 320.04 Appearance Colorless and crystalline Melting point 109.5 °C (229.1 °F (109.5 °C))
Boiling point 350 °C (662 °F (350 °C))
Solubility in water 0.09 mg/L log P 6.02 (octanol-water) Vapor pressure 1.35×10−6 mm Hg kH 6.6×10−6 atm ∙ m³/mol Atmospheric OH rate constant 4.34×10−12 cm³/molecule ∙ s Pharmacology Elimination
half-life4 days in air; 160 days in anaerobic soil; when in water (hydrolysis), depends on various factors (one or more decades in rivers and lakes, 190 years at 27 °C and pH 3–5)[1] Related compounds Related compounds DDE, DDT, mitotane, perthane  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dichlorodiphenyldichloroethane (DDD) is an organochlorine insecticide that is slightly irritating to the skin.[2] DDD is a metabolite of DDT.[3] DDD is colorless and crystalline[4]; it is closely related chemically and is similar in properties to DDT, but it is considered to be less toxic to animals than is DDT.[5] The molecular formula for DDD is (ClC6H4)2CHCHCl2 or C14H10Cl4, whereas the formula for DDT is (ClC6H4)2CHCCl3 or C14H9Cl5.
DDD is in the “Group B2” classification, meaning that it is a probable human carcinogen. This is based on an increased incidence of lung tumors in male and female mice, liver tumors in male mice, and thyroid tumors in male rats. Further basis is that DDD is so similar to and is a metabolite of DDT, another probable human carcinogen.[3]
DDD is no longer registered for agricultural use in the United States, but the general population continues to be exposed to it due to its long persistence time. The primary source of exposure is oral ingestion of food.[1]
1946 is the date of the earliest recorded use in English of the abbreviation “DDD” to stand for dichlorodiphenyldichloroethane, as far as could be determined.[4]
Contents
Table of names
The following are synonyms for DDD:
Systematic Names Superlist Names Other Names Benzene, 1,1'-(2,2-
dichloroethylidene)
bis(4-chloro- (9CI)4,4'-DDD 1,1'-(2,2-Dichloroethylidene)bis
(4-chlorobenzene)Ethane, 1,1-
dichloro-2,2-bis(p-
chlorophenyl)-Benzene, 1,1'-(2,2-
dichloroethylidene)bis(4-chloro-1,1-Bis(4-chlorophenyl)-2,2-
dichloroethaneTDE DDD 1,1-Bis(p-chlorophenyl)-2,2-
dichloroethanep,p'-TDE DDD, p,p'- 1,1-Dichloor-2,2-bis(4-chloor fenyl)-ethaan (Dutch) Dichlorodiphenyldichloroethane 1,1-Dichlor-2,2-bis(4-chlor-
phenyl)-aethan (German)RCRA waste number U060 1,1-Dichloro-2,2-bis(4-
chlorophenyl)-ethane (French)TDE 1,1-Dichloro-2,2-bis(4-
chlorophenyl)ethaneTetrachlorodiphenylethane 1,1-Dichloro-2,2-bis(p-
chlorophenyl)ethanep,p'-TDE 1,1-Dichloro-2,2-bis
(parachlorophenyl)ethane1,1-Dichloro-2,2-di(4-
chlorophenyl)ethane1,1-Dicloro-2,2-bis(4-cloro-fenil)-
etano (Italian)2,2-Bis(4-chlorophenyl)-1,1-
dichloroethane2,2-Bis(p-chlorophenyl)-1,1-
dichloroethane4,4' DDD 4,4-DDD 4,4'-
Dichlorodiphenyldichloroethane4-05-00-01884 (Beilstein Handbook Reference) AI3-04225 Benzene, 1,1'-(2,2-
dichloroethylidene)bis[4-chloro-BRN 1914072 CCRIS 573 Caswell No. 307 DDD analogue DDD in whole water sample Dichlorodiphenyl dichloroethane Dichlorodiphenyldichlorethane Dilene EINECS 200-783-0 ENT 4,225 EPA Pesticide Chemical Code 029101 Ethane, 1,1-dichloro-2,2-bis(p-
chlorophenyl)-HEPT HSDB 285 ME-1700 Me-700 NCI-C00475 NSC 8941 OMS 1078 para-para DDD para,para'-DDD para,para'-
Dichlorodiphenyldichloroethanep,p-DDD p,p'-DDD p,p'-Dichlorodiphenyl-2,2-dichloroethylene p,p'-Dichlorodiphenyldichloroethane Rhothane Rhothane D-3 Rothane Rothane WP-50 References
- “Chemicals: Dichlorodiphenyldichloroethane.” The Comparative Toxicogenomics Database. Mount Desert Island Biological Laboratory, 11 Apr. 2007 <http://ctd.mdibl.org/detail.go?type=chem&acc=D003632>.
- “Data From SRC PhysProp Database.” SRC PhysProp Database. Syracuse Research Corporation. 11 Apr. 2007 <http://esc.syrres.com/interkow/webprop.exe?CAS=72-54-8>.
- “DDD.” Hazardous Substances Data Bank. United States National Library of Medicine. 25 Apr. 2007 <http://toxmap.nlm.nih.gov/toxmap/main/chemPage.jsp?chem=4,4-DICHLORODIPHENYLDICHLOROETHANE then click “Env. Fate / Exposure”>.
- “DDD—RN: 72-54-8.” ChemIDplus Lite Record. 9 Sept. 2004. United States National Library of Medicine Specialized Information Services. 11 Apr. 2007 <http://chem2.sis.nlm.nih.gov/chemidplus/direct.jsp?regno=72-54-8>.
- Guralnik, David B., Editor in Chief. “DDD.” Webster’s New World Dictionary of the American Language. Second College Edition. New York, NY: Prentice Hall Press, 1986. ISBN 0-671-41809-2 (indexed), ISBN 0-671-41807-6 (plain edge), ISBN 0-671-41811-4 (pbk.), and ISBN 0-671-47035-3 (LeatherKraft).
- Mish, Frederick C., Editor in Chief. “DDD.” Webster’s Ninth New Collegiate Dictionary. 9th ed. Springfield, MA: Merriam-Webster Inc., 1985. ISBN 0-87779-508-8, ISBN 0-87779-509-6 (indexed), and ISBN 0-87779-510-X (deluxe).
- “p,p'-DDD.” Substance Registry System. 1 Feb. 2006. United States Environmental Protection Agency. 11 Apr. 2007 <http://iaspub.epa.gov/srs/srs_proc_qry.navigate?P_SUB_ID=4937>.
- “p,p'-Dichlorodiphenyl dichloroethane (DDD) (CASRN 72-54-8).” Integrated Risk Information System. 25 Jan. 2007. United States Environmental Protection Agency. 23 Apr. 2007 <http://www.epa.gov/iris/subst/0347.htm>.
Notes
- ^ a b “DDD.” Hazardous Substances Data Bank. United States National Library of Medicine. 25 Apr. 2007 <http://toxmap.nlm.nih.gov/toxmap/main/chemPage.jsp?chem=4,4-DICHLORODIPHENYLDICHLOROETHANE then click “Env. Fate / Exposure”>.
- ^ Merck Index, 11th ed, p482
- ^ a b “p,p'-Dichlorodiphenyl dichloroethane (DDD) (CASRN 72-54-8).” Integrated Risk Information System. 25 Jan. 2007. United States Environmental Protection Agency. 23 Apr. 2007 <http://www.epa.gov/iris/subst/0347.htm>.
- ^ a b Mish, Frederick C., Editor in Chief. “DDD.” Webster’s Ninth New Collegiate Dictionary. 9th ed. Springfield, MA: Merriam-Webster Inc., 1985.
- ^ Guralnik, David B., Editor in Chief. “DDD.” Webster’s New World Dictionary of the American Language. Second College Edition. New York, NY: Prentice Hall Press, 1986.
External links
- TOXMAP Chemical Page for DDD
- MSDS for rhothane (DDD) provided by the Physical and Theoretical Chemistry Laboratory of the University of Oxford
Categories:- Organochloride insecticides
- Persistent organic pollutants
- Aromatic compounds
Wikimedia Foundation. 2010.