- Xanthone
-
Xanthone 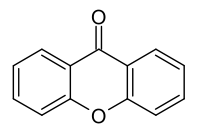 9H-xanthen-9-oneOther names9-oxo-xanthene
9H-xanthen-9-oneOther names9-oxo-xanthene
diphenyline ketone oxideIdentifiers CAS number 90-47-1 
PubChem 7020 ChemSpider 6753 
ChEBI CHEBI:37647 
ChEMBL CHEMBL186784 
Jmol-3D images Image 1 - O=C1c3c(Oc2c1cccc2)cccc3
Properties Molecular formula C13H8O2 Molar mass 196.19 g/mol Appearance off-white solid Melting point 174 °C, 447 K, 345 °F
Boiling point 351 °C, 624 K, 664 °F
Solubility in water sl. sol. in hot water Hazards R-phrases R36/37/38 S-phrases S26 S37[1] Related compounds Related compounds xanthene  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Xanthone is an organic compound with the molecular formula C13H8O2. It can be prepared by the heating of phenyl salicylate.[2] In 1939, xanthone was introduced as an insecticide and it currently finds uses as ovicide for codling moth eggs and as a larvicide.[3] Xanthone is also used in the preparation of xanthydrol, which is used in the determination of urea levels in the blood.
Xanthone derivatives
The chemical structure of xanthone forms the central core of a variety of naturally occurring organic compounds, such as mangostin, which are sometimes collectively referred to as xanthones or xanthonoids.[4] Over 200 xanthones have been identified. Xanthones are natural constituents of plants in the families Bonnetiaceae and Clusiaceae and are found in some species in the family Podostemaceae.[5] Many of these xanthones are found in the pericarp of the mangosteen fruit (Garcinia mangostana), which can be found in the region of Southeast Asia.
Synthetic derivatives of xanthone can be added during the polymerization of polyester, to form a plastic that has a greater resistance to degradation by ultraviolet light.[citation needed] The most useful derivative is tetrahydroxyxanthone. Polyester film can be used for the production of third generation printed solar cells, to make them a cost effective alternative to silica-based solar energy generation. It was originally intended that the additive be used for polyester greenhouses in hot climates, where the plastic would degrade after a few years from UV exposure. The xanthone-treated product has an extended useful lifetime of ten years instead of three.
See also
References
- ^ MSDS from AlphaAesar
- ^ Organic Syntheses, Coll. Vol. 1, p.552 (1941) - preparation of xanthone
- ^ Steiner, L. F. and S. A. Summerland. 1943. Xanthone as an ovicide and larvicide for the codling moth. Journal of economic entomology 36, 435-439.
- ^ PubMed.gov - journal articles on xanthones
- ^ *Angiosperm Phylogeny Group (2003). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399-436 (Available online: Abstract | Full text (HTML) | Full text (PDF))
Categories:- Insecticides
- Xanthones
Wikimedia Foundation. 2010.
