- Cyfluthrin
-
Cyfluthrin 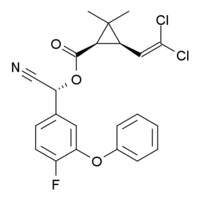 [(R)-cyano-[4-fluoro-3-(phenoxy)phenyl]methyl] (1R,3R)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
[(R)-cyano-[4-fluoro-3-(phenoxy)phenyl]methyl] (1R,3R)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylateIdentifiers CAS number 68359-37-5 
PubChem 50153 ChemSpider 45482 
UNII SCM2QLZ6S0 
KEGG C10982 
ATC code P03,QP53AC12 Jmol-3D images Image 1 - Cl/C(Cl)=C/[C@H]3[C@@H](C(=O)O[C@@H](C#N)c2ccc(F)c(Oc1ccccc1)c2)C3(C)C
- InChI=1S/C22H18Cl2FNO3/c1-22(2)15(11-19(23)24)20(22)21(27)29-18(12-26)13-8-9-16(25)17(10-13)28-14-6-4-3-5-7-14/h3-11,15,18,20H,1-2H3/t15-,18-,20-/m0/s1

Key: QQODLKZGRKWIFG-QSFXBCCZSA-N
InChI=1/C22H18Cl2FNO3/c1-22(2)15(11-19(23)24)20(22)21(27)29-18(12-26)13-8-9-16(25)17(10-13)28-14-6-4-3-5-7-14/h3-11,15,18,20H,1-2H3/t15-,18-,20-/m0/s1
Key: QQODLKZGRKWIFG-QSFXBCCZBF
Properties Molar mass 434.288 Melting point 60 °C
Solubility in water 2 μg/L  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyfluthrin' is a synthetic pyrethroid derivative that is used as an insecticide in restricted use pesticide and common household pesticide. It is a complex organic compound, and the commercial product is sold as a mixture of isomers. Like most pyrethroids, it is highly toxic to fish, invertebrates, and insects, but it is far less toxic to humans.[1] It is generally supplied as a 10-25% liquid concentrate for commercial use and is diluted prior to spraying onto agricultural crops and outbuildings.
Safety
In rats, the LD50's are 500, 800 (oral), and 600 (skin) mg/kg.[1]
Excessive exposure can cause nausea, headache, muscle weakness, salivation, shortness of breath and seizures. In humans, it is deactivated by enzymatic hydrolysis to several carboxylic acid metabolites, whose urinary excretion half-lives are in a range of 5–7 hours. Worker exposure to the chemical can be monitored by measurement of the urinary metabolites, while severe overdosage may be confirmed by quantification of cyfluthrin in blood or plasma.[2]
Health and safety risks are controlled by Right to know laws that exist in most developed countries. Cyfluthrin is regulated in the US by the EPA.[3]
References
- ^ a b Robert L. Metcalf “Insect Control” in Ullmann’s Encyclopedia of Industrial Chemistry” Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a14_263
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 388-389.
- ^ "Pyrethroids and Pyrethrins". United States Environmental Protection Agency. http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html.

This article about an organic compound is a stub. You can help Wikipedia by expanding it.
