- Permethrin
-
Permethrin 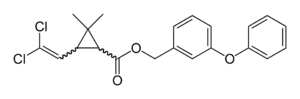 3-Phenoxybenzyl
3-Phenoxybenzyl
(1RS)-cis,trans-3-(2,2-dichlorovinyl)
-2,2-dimethylcyclopropanecarboxylateIdentifiers CAS number 52645-53-1 
PubChem 40326 ChemSpider 36845 
UNII 509F88P9SZ 
DrugBank DB04930 KEGG C14388 
ChEBI CHEBI:34911 
ChEMBL CHEMBL1525 
ATC code P03,QP53AC04 Jmol-3D images Image 1 - Cl/C(Cl)=C/C3C(C(=O)OCc2cccc(Oc1ccccc1)c2)C3(C)C
- InChI=1S/C21H20Cl2O3/c1-21(2)17(12-18(22)23)19(21)20(24)25-13-14-7-6-10-16(11-14)26-15-8-4-3-5-9-15/h3-12,17,19H,13H2,1-2H3

Key: RLLPVAHGXHCWKJ-UHFFFAOYSA-N
InChI=1/C21H20Cl2O3/c1-21(2)17(12-18(22)23)19(21)20(24)25-13-14-7-6-10-16(11-14)26-15-8-4-3-5-9-15/h3-12,17,19H,13H2,1-2H3
Key: RLLPVAHGXHCWKJ-UHFFFAOYAS
Properties Molecular formula C21H20Cl2O3 Molar mass 391.28 g/mol Appearance colourless crystals Density 1.19 g/cm³, solid Melting point 34 °C, 307 K, 93 °F
Boiling point 200 °C, 473 K, 392 °F
Solubility in water Insoluble (5.5 x 10-3 ppm) Hazards MSDS External MSDS Main hazards Irritating to skin and eyes,
damaging to lungsRelated compounds Related pyrethroids Bifenthrin
Deltamethrin (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Permethrin is a common synthetic chemical, widely used as an insecticide, acaricide, and insect repellent. It belongs to the family of synthetic chemicals called pyrethroids and functions as a neurotoxin, affecting neuron membranes by prolonging sodium channel activation. It is not known to rapidly harm most mammals or birds, but is dangerously toxic to cats[1][2] and fish. In general, it has a low mammalian toxicity and is poorly absorbed by skin.[3]
In medicine, permethrin is a first-line treatment for scabies; a 5% (w/w) cream is marketed by Johnson & Johnson under the name Lyclear. In nordic countries it is marketed under trade name Nix, often available over the counter.
Contents
Uses
Permethrin is used:
- as an insecticide
- in agriculture, to protect crops
- in agriculture, to kill livestock parasites
- for industrial/domestic insect control
- as an insect repellent or insect screen
- in timber treatment
- as a personal protective measure (cloth impregnant, used primarily for US military uniforms and mosquito nets)
- in pet flea preventative collars or treatment.
Pest control
In agriculture, permethrin is mainly used on cotton, wheat, maize, and alfalfa crops. Its use is controversial because, as a broad-spectrum chemical, it kills indiscriminately; as well as the intended pests, it can harm beneficial insects including honey bees, and aquatic life.[4]
Permethrin kills ticks on contact with treated clothing. A method of reducing deer tick populations in terms of rodent vectors involves utilizing biodegradable cardboard tubes stuffed with permethrin-treated cotton. Mice collect the cotton for lining their nests. Permethrin on the cotton instantly kills any immature ticks that are feeding on the mice. It is important to put the tubes where mice will find them, such as in dense, dark brush, or at the base of a log; mice are unlikely to gather cotton from an open lawn.
Permethrin is used in tropical areas to prevent mosquito-borne disease such as dengue fever and malaria. Mosquito nets used to cover beds may be treated with a solution of permethrin. This increases the effectiveness of the bed net by killing parasitic insects before they are able to find gaps or holes in the net. Military personnel training in malaria-endemic areas may be instructed to treat their uniforms with permethrin, as well. An application should last several washes.
Personal healthcare
Permethrin is used on humans to eradicate parasites such as head lice and mites responsible for scabies; the common prescription is Permethrin with 5% concentration for scabies, and OTC (over-the-counter) treatment for head lice/crabs is usually permethrin with 1% concentration. However, the British National Formulary states that permethrin has low efficacy in eradicating head lice.
Permethrin is also used in industrial and domestic settings to control pests such as ants and termites.
Stereochemistry
Permethrin has four stereoisomers (two enantiomeric pairs), arising from the two stereocentres in the cyclopropane ring. The trans enantiomeric pair is known as transpermethrin.
-
one cis enantiomer
Toxicology and safety
Permethrin acts as a neurotoxin, slowing down the nervous system through binding to sodium channels. This action is negatively correlated to temperature, thus, in general, showing more acute effects on cold-blooded animals (insects, fish, frogs...) over warm-blooded animals (mammals and birds):
- Permethrin is extremely toxic to fish and aquatic life in general, so extreme care must be taken when using products containing permethrin near water sources.
- Permethrin is also highly toxic to cats, and flea and tick-repellent formulas intended and labeled for (the more resistant) dogs may contain permethrin and cause feline permethrin toxicosis in cats.[5]
- Very high doses will have tangible neurotoxic effects on mammals and birds, including human beings.
Permethrin is listed as a "restricted use" substance by the United States Environmental Protection Agency[6] due to its high toxicity to aquatic organisms.[7]
Due to high toxicity for aquatic life, permethrin and permethrin-contaminated water should be properly disposed of. Degradation is quick and should the chemical be disposed of far from any aquatic life, the negative effects would be minimized. In a non-industrial context, the contaminant may be placed in direct sunlight to induce photodegradation. Contaminated water exposed to direct sunlight will be cleared of the permethrin and any known pollutant subproducts after a few hours.
Human exposure
According to the Connecticut Department of Public Health, permethrin "has low mammalian toxicity, is poorly absorbed through the skin and is rapidly inactivated by the body. Skin reactions have been uncommon."[8]
Excessive exposure to permethrin can cause nausea, headache, muscle weakness, excessive salivation, shortness of breath, and seizures. Worker exposure to the chemical can be monitored by measurement of the urinary metabolites, while severe overdosage may be confirmed by measurement of permethrin in serum or blood plasma.[9]
Permethrin does not present any notable genotoxicity or immunotoxicity in humans and farm animals, but is classified by the United States Environmental Protection Agency (EPA) as a likely human carcinogen, based on reproducible studies in which mice fed permethrin developed liver and lung tumors.[10] Carcinogenic action in nasal mucosal cells due to inhalation exposure is suspected, due to observed genotoxicity in human tissue samples, and in rat livers the evidence of increased pre-neoplastic lesions raises concern over oral exposure.[11][12]
Studies by Bloomquist et al., 2002 [13] suggested a link of permethrin exposure to Parkinson's disease, including very small (per kg.) exposures:
- 2002 study - "Our studies have documented low-dose effects of permethrin, doses below one-one thousandth of a lethal dose for a mouse, with effects on those brain pathways [that are] involved in Parkinson's Disease [...] We have found effects consistent with a pre-parkinsonsian condition, but not yet full-blown parkinsonism." [14][15]
However a more recent 2007 study by the same researcher concluded that there was "little hazard to humans"
- 2007 study - "long-term, low-dose exposure to permethrin alone did not cause signs of neurotoxicity to striatal dopaminergic neural terminals, or enhance the effects of MPTP. We conclude that, under typical use conditions, permethrin poses little Parkinsonian hazard to humans, including when impregnated into clothing for control of biting flies"[16]
A 2006 study in South Africa, found residues of permethrin in breast milk, together with DDT, in an area that experienced DDT treatment for malaria control, as well as the use of pyrethroids in small-scale agriculture.[17]
Domestic animals
Permethrin is toxic to cats. Many cats die after being given flea treatments intended for dogs, or by contact with dogs having recently been treated with permethrin.[18]
See also
References
- ^ "Permethrin Hazards For Cats". ASPCA National Animal Poison Control Center. http://www.vetprof.com/clientinfo/permethrincats.html.
- ^ Franny Syufy. "Cat Flea Control Products Warning". About.com. http://cats.about.com/cs/healthissues/a/permethrin.htm.
- ^ "Permethrin". Pmep.cce.cornell.edu. 1986-04-16. http://pmep.cce.cornell.edu/profiles/extoxnet/metiram-propoxur/permethrin-ext.html. Retrieved 2011-01-05.
- ^ R. H. Ian (1989). "Aquatic organisms and pyrethroids". Pesticide Science 27 (4): 429–457. doi:10.1002/ps.2780270408.
- ^ "report "Cats 'killed by flea treatment'"". BBC News. 2007-11-10. http://news.bbc.co.uk/1/low/uk/7088397.stm. Retrieved 2011-01-05.
- ^ Environmental Protection Agency. "Restricted Use Products (RUP) Report: Six Month Summary List". http://www.epa.gov/opprd001/rup/rup6mols.htm. Retrieved 1 December 2009.
- ^ Environmental Protection Agency. "Permethrin Facts (RED Fact Sheet)". http://www.epa.gov/oppsrrd1/REDs/factsheets/permethrin_fs.htm#uses. Retrieved 2 September 2011.
- ^ Kirby C. Stafford III (February 1999). "Tick Bite Prevention". Connecticut Department of Public Health. http://www.dph.state.ct.us/BCH/infectiousdise/tickborne/tick.htm#Permethrin.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1215-1216.
- ^ Permethrin Facts, US EPA, June 2006.
- ^ M. Tisch, P. Schmezer, M. Faulde, A. Groh and H. Maier (2002). "Genotoxicity studies on permethrin, DEET and diazinon in primary human nasal mucosal cells". European Archives of Oto-Rhino-Laryngology 259 (3): 150–153. doi:10.1007/s004050100406.
- ^ K. Hakoi, R. Cabral, T. Hoshiya, R. Hasegawa, T. Shirai and N. Ito (1992). "Analysis of carcinogenic activity of some pesticides in a medium-term liver bioassay in the rat". Teratogenesis, Carcinogenesis, and Mutagenesis 12 (6): 269–276. doi:10.1002/tcm.1770120605.
- ^ [1] Neurotoxicology. 2002 Oct;23(4-5):537-44.
- ^ [2] BBC News, March 2006.
- ^ [3] Virginia Tech, March 2003.
- ^ Kou, J; Bloomquist, JR (2007). "Neurotoxicity in murine striatal dopaminergic pathways following long-term application of low doses of permethrin and MPTP.". Toxicol Lett 171 (3): 154–161. doi:10.1016/j.toxlet.2007.05.005. PMID 17597311.
- ^ Bouwman, H; Sereda, B; Meinhardt, HM (2006). "Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria-endemic area in South Africa". Environmental Pollution 144 (3): 902–917. doi:10.1016/j.envpol.2006.02.002. PMID 16564119.
- ^ Linnett, PJ (2008). "Permethrin toxicosis in cats". Australian Veterinary Journal 86 (1–2): 32–35. doi:10.1111/j.1751-0813.2007.00198.x. PMID 18271821.
External links
- Permethrin Technical Fact Sheet - National Pesticide Information Center
- Permethrin General Fact Sheet - National Pesticide Information Center
- Permethrin-treated Clothing Hot Topic - National Pesticide Information Center
- "Health Effects of Permethrin-Impregnated Army Battle-Dress Uniforms", National Research Council (1994, US)
- "Permethrin summary report", Committee for Veterinary Products, European Agency for the Evaluation of Medical Products (1998, EU(
- Pesticide link to Parkinson Disease
- Permethrin Pesticide Information Profile - Extension Toxicology Network
- Permethrin chemical data
Human lice and pediculosis Species Infestation Treatment Other terms of interest M: IFT
helm,arth (acar)
helm, arth (lice), zoon
helm, arth
Ectoparasiticides / arthropod (P03A) Insecticide/pediculicide Acaricide/miticide/scabicide PyrethrinesOther/ungroupedM: IFT
helm,arth (acar)
helm, arth (lice), zoon
helm, arth
Categories:- Insecticides
- Antiparasitic agents
- Household chemicals
- Organochlorides
- Endocrine disruptors
- Phenol ethers
- Pyrethroids
- Acaricides
Wikimedia Foundation. 2010.
