- Benzyl benzoate
-
Benzyl benzoate 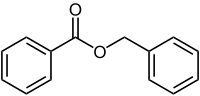
Identifiers CAS number 120-51-4 
PubChem 2345 ChemSpider 13856959 
UNII N863NB338G 
DrugBank DB02775 KEGG D01138 
ChEBI CHEBI:41237 
ChEMBL CHEMBL1239 
ATC code P03,QP53AX11 Jmol-3D images Image 1 - O=C(OCc1ccccc1)c2ccccc2
Properties Molecular formula C14H12O2 Molar mass 212.24 g mol−1 Appearance Colorless liquid Density 1.12 g/cm3 Melting point 18 °C, 291 K, 64 °F
Boiling point 323 °C, 596 K, 613 °F
Hazards MSDS Oxford MSDS EU classification Harmful (Xn) Flash point 158 °C (316 °F) (closed cup)  benzoate (verify) (what is:
benzoate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Benzyl benzoate is the ester of benzyl alcohol and benzoic acid, with the formula C6H5CH2O2CC6H5. This easily prepared compound has a variety of uses.
Contents
Synthesis
This colorless liquid is formally the condensation product of benzoic acid and benzyl alcohol. It can also be generated from benzaldehyde by the Tishchenko reaction.[1]
Uses
Benzyl benzoate, as a topical solution, may be used as an antiparasitic insecticide to kill the mites responsible for the skin condition scabies,[2] for example as a combination drug of benzyl benzoate/disulfiram.[3]
It has other uses:
- a fixative in fragrances to improve the stability and other characteristics of the main ingredients
- a food additive in artificial flavors[citation needed]
- a plasticizer in cellulose and other polymers
- a solvent for various chemical reactions
- a treatment for sweet itch in horses[4]
- a treatment for scaly leg mites in chickens
List of plants that contain the chemical
Compendial status
- British Pharmacopoeia 2009 [7]
- Japanese Pharmacopoeia 15
References
- ^ Kamm, O.; Kamm, W. F. (1941), "Benzyl benzoate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0104; Coll. Vol. 1: 104
- ^ "Benzyl Benzoate". Patient UK. 2005-02-10. http://www.patient.co.uk/showdoc/30002528/.
- ^ Landegren J, Borglund E, Storgårds K (1979). "Treatment of scabies with disulfiram and benzyl benzoate emulsion: a controlled study". Acta Derm. Venereol. 59 (3): 274–6. PMID 87094.
- ^ National Sweet Itch Centre
- ^ Woerdenbag, Herman J. et al.; Windono, Tri; Bos, Rein; Riswan, Sudarsono; Quax, Wim J. (2004). "Composition of the essential oils of Kaempferia rotunda L. and Kaempferia angustifolia Roscoe rhizomes from Indonesia". Flavour and Fragrance Journal 19 (2): 145–148. doi:10.1002/ffj.1284.
- ^ a b Nugroho, Bambang W. et al. (1996). "Insecticidal constituents from rhizomes of Zingiber cassumunar and Kaempferia rotunda". Phytochemistry 41 (1): 129–132. doi:10.1016/0031-9422(95)00454-8.
- ^ British Pharmacopoeia Commission Secretariat (2009). "Index (BP 2009)". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf. Retrieved 3 July 2009.
Ectoparasiticides / arthropod (P03A) Insecticide/pediculicide Chlorine-containing productsAcaricide/miticide/scabicide PyrethrinesSulfur-containing productsChlorine-containing productsBenzyl benzoate#Other/ungroupedM: IFT
helm,arth (acar)
helm, arth (lice), zoon
helm, arth
Categories:- Benzoates
- Insecticides
- Ester solvents
- Plasticizers
Wikimedia Foundation. 2010.

