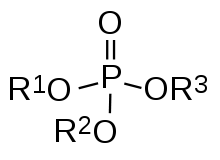
- Organophosphate
-
An organophosphate (sometimes abbreviated OP) is the general name for esters of phosphoric acid. Phosphates are probably the most pervasive organophosphorus compounds. Many of the most important biochemicals are organophosphates, including DNA and RNA as well as many cofactors that are essential for life. Organophosphates are the basis of many insecticides, herbicides, and nerve gases. The EPA lists organophosphates as very highly acutely toxic to bees, wildlife, and humans.[1] Recent studies suggest a possible link to adverse effects in the neurobehavioral development of fetuses and children, even at very low levels of exposure. Organophosphates are widely used as solvents, plasticizers, and EP additives.
Organophosphates are widely employed both in natural and synthetic applications because of the ease with which organic groups can be linked together. Being a triprotic acid, phosphoric acid can form triesters whereas carboxylic acids only form monoesters. Esterification entails the attachment of organic groups to phosphorus through oxygen linkers. The precursors to such esters are alcohols. Encompassing many thousands of natural and synthetic compounds, alcohols are diverse and widespread.
- OP(OH)3 + ROH → OP(OH)2(OR) + H2O
- OP(OH)2(OR) + R'OH → OP(OH)(OR)(OR') + H2O
- OP(OH)(OR)(OR') + R"OH → OP(OR)(OR')(OR") + H2O
The phosphate esters bearing OH groups are acidic and partially deprotonated in aqueous solution. For example, DNA and RNA are polymers of the type [PO2(OR)(OR')-]n. Polyphosphates also form esters; an important example of an ester of a polyphosphate is ATP, which is the monoester of triphosphoric acid (H5P3O10).
Alcohols can be detached from phosphate esters by hydrolysis, which is the reverse of the above reactions. For this reason, phosphate esters are common carriers of organic groups in biosynthesis.
Contents
Organophosphate pesticides
In health, agriculture, and government, the word "organophosphates" refers to a group of insecticides or nerve agents acting on the enzyme acetylcholinesterase (the pesticide group carbamates also act on this enzyme, but through a different mechanism). The term is used often to describe virtually any organic phosphorus(V)-containing compound, especially when dealing with neurotoxic compounds. Many of the so-called organophosphates contain C-P bonds. For instance, sarin is O-isopropyl methylphosphonofluoridate, which is formally derived from phosphorous acid (HP(O)(OH)2), not phosphoric acid (P(O)(OH)3). Also, many compounds which are derivatives of phosphinic acid are used as neurotoxic organophosphates.
Organophosphate pesticides (as well as sarin and VX nerve agent) irreversibly inactivate acetylcholinesterase, which is essential to nerve function in insects, humans, and many other animals. Organophosphate pesticides affect this enzyme in varied ways, and thus in their potential for poisoning. For instance, parathion, one of the first OPs commercialized, is many times more potent than malathion, an insecticide used in combatting the Mediterranean fruit fly (Med-fly) and West Nile Virus-transmitting mosquitoes.
Organophosphate pesticides degrade rapidly by hydrolysis on exposure to sunlight, air, and soil, although small amounts can be detected in food and drinking water. Their ability to degrade made them an attractive alternative to the persistent organochloride pesticides, such as DDT, aldrin and dieldrin. Although organophosphates degrade faster than the organochlorides, they have greater acute toxicity, posing risks to people who may be exposed to large amounts (see the Toxicity section below).
Commonly used organophosphates have included parathion, malathion, methyl parathion, chlorpyrifos, diazinon, dichlorvos, phosmet, fenitrothion[2] tetrachlorvinphos, and azinphos methyl. Malathion is widely used in agriculture, residential landscaping, public recreation areas, and in public health pest control programs such as mosquito eradication.[3] In the US, it is the most commonly used organophosphate insecticide.[4] Forty organophosphate pesticides are registered in the U.S., with at least 73 million pounds used in agricultural and residential settings.[5]
They are of concern to both scientists and regulators because they work by irreversibly blocking an enzyme that’s critical to nerve function in both bugs and people. Even at relatively low levels, organophosphates may be most hazardous to the brain development of fetuses and young children. The EPA banned most residential uses of organophosphates in 2001, but they are still sprayed agriculturally on fruits and vegetables. They’re also used to control pests like mosquitos in public spaces such as parks. They can be absorbed through the lungs or skin or by eating them on food.[6]
Organophosphates as nerve agents
History of nerve agents
Early pioneers in the field include Jean Louis Lassaigne (early 19th century) and Philippe de Clermont (1854). In 1932, German chemist Willy Lange and his graduate student, Gerde von Krueger, first described the cholinergic nervous system effects of organophosphates, noting a choking sensation and a dimming of vision after exposure. This discovery later inspired German chemist Gerhard Schrader at company IG Farben in the 1930s to experiment with these compounds as insecticides. Their potential use as chemical warfare agents soon became apparent, and the Nazi government put Schrader in charge of developing organophosphate (in the broader sense of the word) nerve gases. Schrader's laboratory discovered the G series of weapons, which included Sarin, Tabun, and Soman. The Nazis produced large quantities of these compounds, though did not use them during World War II. British scientists experimented with a cholinergic organophosphate of their own, called diisopropylfluorophosphate (DFP), during the war. The British later produced VX nerve agent, which was many times more potent than the G series, in the early 1950s, almost 20 years after the Germans had discovered the G series.
After World War II, American companies gained access to some information from Schrader's laboratory, and began synthesizing organophosphate pesticides in large quantities. Parathion was among the first marketed, followed by malathion and azinphosmethyl. The popularity of these insecticides increased after many of the organochlorine insecticides like DDT, dieldrin, and heptachlor were banned in the 1970s.
Structural features of organophosphates
Effective organophosphates have the following structural features:
- A terminal oxygen connected to phosphorus by a double bond, i.e. a phosphoryl group
- Two lipophilic groups bonded to the phosphorus
- A leaving group bonded to the phosphorus, often a halide
Terminal oxygen vs. terminal sulfur
Thiophosphoryl compounds, those bearing the P=S functionality, are much less toxic than related phosphoryl derivatives, which include sarin, VX and tetraethyl pyrophosphate. Thiophosphoryl compounds are not active inhibitors of acetylcholinesterase in either mammals or insects; in mammals, metabolism tends to remove lipophilic side groups from the phosphorus atom while in insects it tends to oxidize the compound, thus removing the terminal sulfur and replacing it with a terminal oxygen, which allows the compound to more efficiently act as an acetylcholinesterase inhibitor.
Fine tuning
Within these requirements, a large number of different lipophilic and leaving groups have been used. The variation of these groups is one means of fine tuning the toxicity of the compound. A good example of this chemistry are the P-thiocyanate compounds which use an aryl (or alkyl) group and an alkylamino group as the lipophilic groups. The thiocyanate is the leaving group.
It was claimed in a German patent that the reaction of 1,3,2,4-dithiadiphosphetane 2,4-disulfides with dialkyl cyanamides formed plant protection agents which contained six membered (P-N=C-N=C-S-) rings. It has been proven in recent times by the reaction of diferrocenyl 1,3,2,4-dithiadiphosphetane 2,4-disulfide (and Lawesson's reagent) with dimethyl cyanamide that, in fact, a mixture of several different phosphorus-containing compounds is formed. Depending on the concentration of the dimethyl cyanamide in the reaction mixture, either a different six membered ring compound (P-N=C-S-C=N-) or a nonheterocylic compound (FcP(S)(NR2)(NCS)) is formed as the major product; the other compound is formed as a minor product.
In addition, small traces of other compounds are also formed in the reaction. It is unlikely that the ring compound (P-N=C-S-C=N-) {or its isomer} would act as a plant protection agent, but (FcP(S)(NR2)(NCS)) compounds can act as nerve poisons in insects.
Organophosphate poisoning
Many organophosphates are potent nerve agents, functioning by inhibiting the action of acetylcholinesterase (AChE) in nerve cells. They are one of the most common causes of poisoning worldwide, and are frequently intentionally used in suicides in agricultural areas. Organophosphorus pesticides can be absorbed by all routes, including inhalation, ingestion, and dermal absorption. Their toxicity is not limited to the acute phase, however, and chronic effects have long been noted. Neurotransmitters such as acetylcholine (which is affected by organophosphate pesticides) are profoundly important in the brain's development, and many OPs have neurotoxic effects on developing organisms, even from low levels of exposure. Other organophosphates are not toxic, yet their main metabolites, such as their oxons are.
Health effects
Chronic Toxicity
Repeated or prolonged exposure to organophosphates may result in the same effects as acute exposure including the delayed symptoms. Other effects reported in workers repeatedly exposed include impaired memory and concentration, disorientation, severe depressions, irritability, confusion, headache, speech difficulties, delayed reaction times, nightmares, sleepwalking and drowsiness or insomnia. An influenza-like condition with headache, nausea, weakness, loss of appetite, and malaise has also been reported.[7]
Low level exposure
Even at relatively low levels organophosphates may be hazardous to human health. The pesticides act on a set of brain chemicals closely related to those involved in ADHD, thus fetuses and young children, where brain development depends on a strict sequence of biological events, may be most at risk.[8] They can be absorbed through the lungs or skin or by eating them on food. According to a 2008 report from the U.S. Department of Agriculture, in a representative sample of produce tested by the agency, 28 percent of frozen blueberries, 20 percent of celery, 27 percent of green beans, 17 percent of peaches, 8 percent of broccoli and 25 percent of strawberries contained traces of organophosphate.[9]
The United States Environmental Protection Agency lists the organophosphate parathion as a possible human carcinogen.[10]
Chronic fatigue is common amongst those who consider their health is affected by pesticides and research from 2003 suggested there was an association between exposure to organophosphates and chronic fatigue symptoms.[11]
A 2007 study linked the organophosphate insecticide chlorpyrifos, which is used on some fruits and vegetables, with delays in learning rates, reduced physical coordination, and behavioral problems in children, especially ADHD.[12]
A 2010 study has found that organophosphate exposure is associated with an increased risk of Alzheimer's disease.[13]
A 2010 study found that each 10-fold increase in urinary concentration of organophosphate metabolites was associated with a 55% to 72% increase in the odds of ADHD in children.[14][15][16] The study found that organophosphate exposure is associated with an increased risk of ADHD in children. Researchers analyzed the levels of organophosphate residues in the urine of more than 1,100 children aged 8 to 15 years old, and found that those with the highest levels of dialkyl phosphates, which are the breakdown products of organophosphate pesticides, also had the highest incidence of ADHD. Overall, they found a 35% increase in the odds of developing ADHD with every 10-fold increase in urinary concentration of the pesticide residues. The effect was seen even at the low end of exposure: children who had any detectable, above-average level of pesticide metabolite in their urine were twice as likely as those with undetectable levels to record symptoms of ADHD.
Another 2010 study found that children who were exposed to organophosphate pesticides while still in their mother's womb were more likely to develop attention disorders years later. The researchers evaluated the children at age 3.5 and 5 years for symptoms of attention disorders and ADHD using maternal reports of child behavior, performance on standardized computer tests, and behavior ratings from examiners. Each tenfold increase in prenatal pesticide metabolites was linked to having five times the odds of scoring high on the computerized tests at age 5, suggesting a greater likelihood of a child having ADHD. The effect appeared to be stronger for boys than for girls.[17]
In 2011 the results of three government-funded studies that charted "everyday" environmental exposures in hundreds of women and their children through pregnancy and into their grade school years was released. Though each study used a somewhat different way to track the pesticide exposures, they all reached strikingly similar conclusions—that many children exposed to higher levels of organophosphates during pregnancy than their peers are more likely to have lower IQs and may have more difficulty focusing on tasks or solving problems. In one study children who were exposed to the highest levels of organophosphates during pregnancy had IQ scores that were an average of 7 points lower than the IQ scores of those with the lowest pesticide exposures. It was found that genetics appear to play a strong role in whether exposure to organophosphates will cause damage. Two studies found that children exposed to higher levels of organophosphate pesticides than their peers were more likely to be diagnosed with attention deficit hyperactivity disorder.[6][18]
Proposals to ban and restrictions of use
According to the non-governmental organisation Pesticide Action Network, the organophosphate parathion is one of the most dangerous pesticides.[19] In the US alone more than 650 agricultural workers have been poisoned since 1966, of which 100 died. In underdeveloped countries many more people have suffered fatal and nonfatal intoxications. The World Health Organization, PAN and numerous environmental organisations propose a general and global ban. Its use is banned or restricted in 23 countries and its import is illegal in a total of 50 countries.[20] Its use was banned in the U.S. in 2000 and it has not been used since 2003.[20]
Other than for agricultural use, the organophosphate diazinon has been banned in the U.S. Agriculturally, more than one million pounds of diazinon were used in California to control agricultural pests in 2000. The areas and crops on which diazinon are most heavily applied are structural pest control, almonds, head lettuce, leaf lettuce and prunes.[21]
In May 2006 the Environmental Protection Agency reviewed the use of the organophosphate dichlorvos and proposed its continued sale, despite concerns over its safety and considerable evidence suggesting it is carcinogenic and harmful to the brain and nervous system, especially in children. Environmentalists charge that the latest decision was the product of backroom deals with industry and political interference.[22]
In 2001 the EPA placed new restrictions on the use of the organophosphates phosmet and azinphos-methyl to increase protection of agricultural workers. The crop uses reported at that time as being phased out in four years included those for almonds, tart cherries, cotton, cranberries, peaches, pistachios, and walnuts. The crops with time-limited registration included apples/crab apples, blueberries, sweet cherries, pears, pine seed orchards, brussels sprouts, cane berries, and the use of azinphos-methyl by nurseries for quarantine requirements.[23] The labeled uses of phosmet include alfalfa, orchard crops (e.g. almonds, walnuts, apples, cherries), blueberries, citrus, grapes, ornamental trees (not for use in residential, park, or recreational areas) and non-bearing fruit trees, Christmas trees and conifers (tree farms), potatoes and peas.[24] Azinphos-methyl has been banned in Europe since 2006.[25][26]
See also
- Organophosphate poisoning
- Activity based protein profiling using organophosphate-containing activity based probes
- Mark Purdey
- Pesticide toxicity to bees
- Toxic Syndrome (Spain)
References
- ^ http://www.epa.gov/pesticides/about/intheworks/clothianidin-registration-status.html#basic
- ^ http://extoxnet.orst.edu/pips/fenitrot.htm
- ^ Malathion for mosquito control, US EPA
- ^ Bonner MR, Coble J, Blair A, et al. (2007). "Malathion Exposure and the Incidence of Cancer in the Agricultural Health Study". American Journal of Epidemiology 166 (9): 1023–34. doi:10.1093/aje/kwm182. PMID 17720683.
- ^ Maugh II, Thomas H. (16 May 2010). "Study links pesticide to ADHD in children". Los Angeles Times. http://articles.latimes.com/2010/may/16/science/la-sci-pesticides-20100517.
- ^ a b http://www.webmd.com/baby/news/20110421/pesticide-exposure-in-womb-linked-to-lower-iq
- ^ http://extoxnet.orst.edu/pips/parathio.htm
- ^ http://versita.metapress.com/content/g4470858487t28u4/
- ^ "Study: ADHD linked to pesticide exposure". CNN. 17 May 2010. http://www.cnn.com/2010/HEALTH/05/17/pesticides.adhd/.
- ^ http://www.epa.gov/iris/subst/0327.htm
- ^ Tahmaz, Soutar and Cherrie. Chronic fatigue and organophosphate pesticides in sheep farming: a retrospective study amongst people reporting to a UK pharmacovigilance scheme. The Annals of Occupational Hygiene (2003) vol. 47 (4) pp. 261-7. http://annhyg.oxfordjournals.org/content/47/4/261.full
- ^ Study Links Organophosphate Insecticide Used on Corn With ADHD. Beyond Pesticides. 5 January 2007.
- ^ Hayden, K. .; Norton, M. .; Darcey, D. .; Ostbye, T. .; Zandi, P. .; Breitner, J. .; Welsh-Bohmer, K. .; Cache County Study, I. . (2010). "Occupational exposure to pesticides increases the risk of incident AD: the Cache County study". Neurology 74 (19): 1524–1530. doi:10.1212/WNL.0b013e3181dd4423. PMC 2875926. PMID 20458069. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2875926.
- ^ http://pediatrics.aappublications.org/cgi/reprint/peds.2009-3058v1.pdf
- ^ http://www.medscape.com/viewarticle/721892
- ^ Klein, Sarah. Study: ADHD linked to pesticide exposure. CNN. 17 May 2010.
- ^ http://ehp03.niehs.nih.gov/article/info%3Adoi%2F10.1289%2Fehp.1002056
- ^ Time. 21 April 2011. http://healthland.time.com/2011/04/21/exposure-to-pesticides-in-pregnancy-can-lower-childrens-iq/.
- ^ S. Kegley, B. Hill, S. Orme. "Parathion - Identification, toxicity, use, water pollution potential, ecological toxicity and regulatory information". Pesticide Action Network. http://pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC35122.
- ^ a b http://pmep.cce.cornell.edu/profiles/insect-mite/mevinphos-propargite/parathion/parath_can_0900.html
- ^ http://www.greatvistachemicals.com/agrochemicals/diazinon.html
- ^ http://www.scientificamerican.com/article.cfm?id=slow-acting
- ^ http://www.icis.com/Articles/2001/11/01/150226/us-epa-restricts-pesticides-azinphos-methyl-phosmet.html
- ^ http://www.epa.gov/espp/litstatus/effects/redleg-frog/2010/phosmet/assessment.pdf
- ^ http://www.chemweek.com/envirotech/regulatory/13435.html
- ^ http://earthjustice.org/news/press/2007/suit-filed-to-speed-phase-out-of-three-deadly-pesticides
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006 Apr;366(1-2):1-13. Epub 2005 December 6. Review. PMID: 16337171.
External links
- ATSDR Case Studies in Environmental Medicine: Cholinesterase Inhibitors, Including Pesticides and Chemical Warfare Nerve Agents U.S. Department of Health and Human Services
- Organophosphates - an article by Frances M Dyro, MD
- Pesticides Action Network, North America
- NYTimes: E.P.A. Recommends Limits On Thousands of Pesticides
- EPA OP pesticide site
Health issues of plastics and Polyhalogenated compounds (PHCs) Plasticizers: Phthalates Miscellaneous plasticizers Monomers Bisphenol A (BPA, in Polycarbonates) · Vinyl chloride (in PVC)Miscellaneous additives incl. PHCs Health issues Miscellanea PVC · Plastic recycling · Plastic bottle · Vinyl chloride · Dioxins · Polystyrene · Styrofoam · PTFE (Teflon) · California Proposition 65 · List of environmental health hazards · Persistent organic pollutant · European REACH regulation · Japan Toxic Substances Law · Toxic Substances Control ActCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Organophosphates
- Antiparasitic agents
Wikimedia Foundation. 2010.
Look at other dictionaries:
organophosphate — [ôr΄gə nō fäs′fāt΄; ôr gan΄ōfäs′fāt΄] n. [ ORGANO + PHOSPHATE] any organic compound containing phosphorus, specif. one used as an insecticide, as malathion … English World dictionary
organophosphate — UK [ɔː(r)ˌɡænəʊˈfɒsˌfeɪt] / US [ɔrˌɡænoʊˈfɑsˌfeɪt] noun [countable] Word forms organophosphate : singular organophosphate plural organophosphates a substance containing phosphorus that is used as a pesticide. Some organophosphates are poisonous … English dictionary
organophosphate — noun Date: 1949 an organophosphorus compound (as a pesticide) • organophosphate adjective … New Collegiate Dictionary
Organophosphate — Phosphorsäureester (auch: Alkylphosphate) sind Ester der ortho Phosphorsäure, die formal oder tatsächlich durch die Reaktion der Säure und Alkoholen unter Abspaltung von Wasser entstehen. Sie können als organische Phosphate/Organophosphate… … Deutsch Wikipedia
organophosphate — or·gan·o·phos·phate ȯr .gan ə fäs .fāt n an organophosphorus compound organophosphate adj * * * or·ga·no·phos·phate (or″gə no fosґfāt) phosphate esterified to organic compounds such as glucose or sorbitol; see organophosphorus … Medical dictionary
Organophosphate poisoning — Classification and external resources Phosphoric acid ICD 10 T … Wikipedia
Organophosphate-induced delayed neuropathy — (OPIDN), also called organophosphate induced delayed polyneuropathy (OPIDP), is a neuropathy caused by killing of neurons in the central nervous system, especially in the spinal cord, as a result of acute or chronic organophosphate poisoning. A… … Wikipedia
organophosphate nerve agent — noun any of a series of nerve agents containing organophosphate compounds first synthesized by German chemists in 1936; in World War II the Germans tested them in concentration camps but not on the battlefield; Iraq is alleged to have used them… … Useful english dictionary
organophosphate — /awr geuh noh fos fayt, awr gan euh /, n. Biochem. any of a variety of organic compounds that contain phosphorus and often have intense neurotoxic activity: originally developed as nerve gases, now widely used as insecticides and fire retardants … Universalium
organophosphate — noun any ester of phosphoric acid or its derivatives, especially one used as an insecticide or herbicide … Wiktionary