- Indoxacarb
-
Indoxacarb 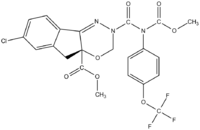 Methyl 7-Chloro-2,5-dihydro-2-
Methyl 7-Chloro-2,5-dihydro-2-
[[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]
4a(3H)-carboxylate
amino]carbonyl]indeno[1,2-e][1,3,4]oxadiazine-Systematic nameMethyl 7-chloro-2-{[(methoxycarbonyl)[4-
(trifluoromethoxy)phenyl]amino]carbonyl}-2H,
carboxylate
3H,4aH,5H-indeno[1,2-e][1,3,4]oxadiazine-4a-Identifiers Abbreviations DPX-MP062 CAS number 173584-44-6 (4aS)  , 144171-61-9
, 144171-61-9PubChem 9936739  , 18772464 (4aR)
, 18772464 (4aR)  , 107720 (4aS)
, 107720 (4aS) 
ChemSpider 8112367  , 11677297 (4aR)
, 11677297 (4aR)  , 96889 (4aS)
, 96889 (4aS) 
UNII 52H0D26MWR UN number UN 3077 KEGG D06316 MeSH Indoxacarb ChEBI CHEBI:38630 ATCvet code QP53 Beilstein Reference 8366683 Jmol-3D images {{#if:COC(=O)N(C(=O)N1COC2(CC3=C(C=CC
(Cl)=C3)C2=N1)C(=O)OC)C1=CC=C(OC
(F)(F)F)C=C1|Image 1- COC(=O)N(C(=O)N1COC2(CC3=C(C=CC
(Cl)=C3)C2=N1)C(=O)OC)C1=CC=C(OC
(F)(F)F)C=C1
- InChI=1S/C22H17ClF3N3O7/c1-33-18(30)21-
10-12-9-13(23)3-8-16(12)17(21)27-28(11-
35-21)19(31)29(20(32)34-2)14-4-6-15(7-5-
14)36-22(24,25)26/h3-9H,10-11H2,1-2H3
Key: VBCVPMMZEGZULK-UHFFFAOYS
A-N
Properties Molecular formula C22H17ClF3N3O7 Molar mass 527.87 Melting point 88.1 C (99% indoxacarb PAI)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Indoxacarb is an oxadiazine pesticide developed by DuPont that acts against lepidopteran larvae. It is marketed under the names Indoxacarb Technical Insecticide, Steward Insecticide and Avaunt Insecticide. It is also used as the active ingredient in DuPont's line of commercial pesticides: Advion and Arilon.[1][2][3]
Indoxacarb is the active ingredient in a number of household insecticides, including cockroach baits, and can remain active after digestion. Its main mode of action is via blocking of nerve sodium channels. It is fairly lipophilic with a Kow of 4.65.[4]
References
- ^ United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances (7505C). Pesticide Fact Sheet. Name of Chemical: Indoxacarb. Reason for Issuance: Conditional Registration. Date Issued: October 30, 2000.
- ^ United States Environmental Protection Agency. Federal Register: Indoxacarb; Pesticide Tolerance. Federal Register: July 11, 2007 (Volume 72, Number 132)
- ^ Commission Directive 2006/10/EC of 27 January 2006 amending Council Directive 91/414/EEC to include forchlorfenuron and indoxacarb as active substances. Official Journal of the European Union 2006-1-28
- ^ "Indoxacarb Insecticide Wipes Out Entire Cockroach Generations". June 23, 2008. http://www.scientificblogging.com/news_releases/indoxacarb_insecticide_wipes_out_entire_cockroach_generations.. Retrieved 2009-12-14.
Further reading
- Lapied, Bruno; Françoise Grolleau and David B Sattelle (January 2001). "Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels". Br J Pharmacol 132 (2): 587–595. doi:10.1038/sj.bjp.0703853. PMC 1572588. PMID 11159709. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1572588.
- Khambay, Bhupinder P.S. (2002). "Pyrethroid Insecticides". Pesticide Outlook 13 (2): 49–54. doi:10.1039/b202996k. http://www.rsc.org/publishing/journals/PO/article.asp?doi=b202996k.
- Moncada, Adriana. Environmental Fate of Indoxacarb. Environmental Monitoring Branch, Department of Pesticide Regulation, State of California. March 6, 2003
- Tillman, P Glynn; Hammes, Glenn G : Sacher, Matthew : Connair, Michael : Brady, E Angela : Wing, Keith D (January 2002). "Toxicity of a formulation of the insecticide indoxacarb to the tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae), and the big-eyed bug, Geocoris punctipes (Hemiptera: Lygaeidae)". Pest-Manag-Sci 58 (1): 92–100. doi:10.1002/ps.426. PMID 11838290. http://grande.nal.usda.gov/ibids/index.php?mode2=detail&origin=ibids_references&therow=701948.
External links
- DuPont Professional Products - Indoxacarb. Updated 2006-08-23 Retrieved 2008-05-12
- DuPont Steward insecticide - FAQs. Updated 20 January 2007. Retrieved 2008-05-12
Categories:- Pesticides
- Ureas
- Methyl esters
- Organofluorides
- COC(=O)N(C(=O)N1COC2(CC3=C(C=CC
Wikimedia Foundation. 2010.
