- Bis(2-ethylhexyl) phthalate
-
Bis(2-ethylhexyl) phthalate 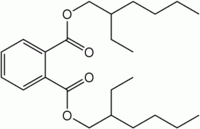 Bis(2-ethylhexyl)phthalate (BEHP)
Bis(2-ethylhexyl)phthalate (BEHP)Identifiers CAS number 117-81-7 
ChemSpider 8040 
UNII C42K0PH13C 
KEGG C03690 
ChEMBL CHEMBL402794 
Jmol-3D images Image 1 - O=C(OCC(CC)CCCC)
C1=CC=CC=C1C(OCC(CC)CCCC)=O
Properties Molecular formula C24H38O4 Molar mass 390.56 Melting point -50°C
Boiling point 385°C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Bis(2-ethylhexyl)phthalate, commonly abbreviated DEHP, is an organic compound with the formula C6H4(C8H17COO)2. It is sometimes called dioctyl phthalate and abbreviated DOP. It is the most important "phthalate," being the diester of phthalic acid and the branched-chain 2-ethylhexanol. This colourless viscous liquid is soluble in oil, but not in water. It possesses good plasticizing properties. Being produced on a massive scale by many companies, it has acquired many names and acronyms, including BEHP and di-2-ethyl hexyl phthalate.
Contents
Production
The process entails the reaction of phthalic anhydride with 2-ethylhexanol:
- C6H4(CO)2O + 2 C8H17OH → C6H4(CO2 C8H17)2 + H2O
Approximately three billion kilograms are produced annually.[1]
Use
Due to its suitable properties and the low cost, DEHP is widely used as a plasticizer in manufacturing of articles made of PVC.[1] Plastics may contain 1% to 40% of DEHP. It is also used as a hydraulic fluid and as a dielectric fluid in capacitors. DEHP also finds use as a solvent in glowsticks.
Environmental exposure
DEHP has a low vapor pressure, but the temperatures for processing PVC articles are often high, leading to release of elevated levels, raising concerns about health risks (see outgassing). It can be absorbed from food and water. Higher levels have been found in milk and cheese. It can also leach into a liquid that comes in contact with the plastic; it extracts faster into nonpolar solvents (e.g. oils and fats in foods packed in PVC). Food and Drug Administration (FDA) therefore permits use of DEHP-containing packaging only for foods that primarily contain water. In soil, DEHP contamination moves very slowly because of its low solubility in water. Therefore, leaching from disposed plastics in landfills is generally slow. The US EPA limits for DEHP in drinking water is 6 ppb. The U.S. agency OSHA's limit for occupational exposure is 5 mg/m3 of air.
Use in medical devices
DEHP has been used as a plasticiser in medical devices such as intravenous tubing and bags, catheters, nasogastric tubes, dialysis bags and tubing, and blood bags and transfusion tubing, and air tubes. For this reason, concern has been expressed about leachates transported into the patient, especially for those requiring extensive infusions, e.g. newborns in intensive care nursery settings, hemophiliacs, and kidney dialysis patients. According to the European Commission Scientific Committee on Health and Environmental Risks (SCHER), exposure to DEHP may exceed the tolerable daily intake in some specific population groups, namely people exposed through medical procedures such as kidney dialysis.[2] The American Academy of Pediatrics has advocated not to use medical devices that can leach DEHP into patients and, instead, to resort to DEHP-free alternatives.[citation needed] In July 2002, the U.S. FDA issued a Public Health Notification on DEHP, stating in part, "We recommend considering such alternatives when these high-risk procedures are to be performed on male neonates, pregnant women who are carrying male fetuses, and peripubertal males" noting that the alternatives were to look for non-DEHP exposure solutions;[3] they mention a database of alternatives.[4] The CBC documentary The Disappearing Male raised concerns about sexual development in male fetal development, miscarriage (as DEHP is a pseudo-estrogen and a hormone modifier found in most plastic products such as PVC, polycarbonate, nearly all cosmetic chemical products, and many others), and as a cause of dramatically lower sperm counts in men. [5]
Metabolism
DEHP hydrolyzes to MEHP (mono-ethylhexyl phthalate) and subsequently to phthalate salts. The released alcohol is susceptible to oxidation to the aldehyde and carboxylic acid.[1]
Effects on living organisms
Smaller penis size and other feminizing links
DEHP metabolites measured from the blood of pregnant women have been significantly associated with the decreased penis width, shorter anogenital distance, and the incomplete descent of testes of their newborn sons, replicating effects identified in animals.[6] Approximately 25% of US women have phthalate levels similar to those in the study.[6]
Obesity
A study on CDC data, published in Environmental Health Perspectives (EHP), "revealed that American men with abdominal obesity or insulin resistance (a precursor to diabetes) were more likely to have high levels of [DEHP and DBP] metabolites in their urine than men without those problems."[7]
Toxicity
The acute toxicity of DEHP is 30g/kg in rats (oral) and 24g/kg in rabbits (dermal).[1] Concerns instead focus on its potential as an endocrine disruptor. Some countries have banned DEHP from toys; see phthalate for legal status.
Cardiotoxicity
A clinically relevant dose and duration of exposure to DEHP has been shown to have a significant impact on the behavior of cardiac cells in culture. This includes an uncoupling effect that leads to irregular rhythms in vitro. This is observed in conjunction with a significant decrease in the amount of gap junctional connexin proteins in cardiomyocytes treated with DEHP.[8][9]
Alternative plasticizers
Manufacturers of flexible PVC articles can choose among several alternative plasticizers offering similar technical properties as DEHP. These alternatives include other phthalates such as DINP, DPHP, DIDP, and non-phthalates, e.g. DINCH and Citrates.
Government and industry response
Taiwan
In October 2009, Consumers' Foundation, Chinese Taipei (CFCT) published test results[10] that found 5 out of the sampled 12 shoes contained over 0.1% of phthalate plasticizer content, including DEHP, which exceeds the government's Toy Safety Standard (CNS 4797). CFCT recommend that users should first wear socks to avoid direct skin contact.
In May 2011, the illegal use of the plasticizer DEHP in clouding agents for use in food and beverages has been reported in Taiwan.[11] An inspection of products initially discovered the presence of plasticizers. As more products were tested, inspectors found more manufacturers using DEHP and DINP.[12] The Department of Health confirmed that contaminated food and beverages had been exported to other countries and regions, which reveals the widespread prevalence of toxic plasticizers.
For more details on this topic, see 2011 Taiwan food scandal.References
- ^ a b c d Peter M. Lorz, Friedrich K. Towae, Walter Enke, Rudolf Jäckh, Naresh Bhargava, Wolfgang Hillesheim "Phthalic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim, 2002.
- ^ "Phthalates in school supplies". GreenFacts Website. http://copublications.greenfacts.org/en/phthalates-school-supplies/. Retrieved 2009-06-10.
- ^ FDA Public Health Notification: PVC Devices Containing the Plasticizer DEHP, USFDA July 12, 2002
- ^ Products for Hazard: DEHP, Sustainable Hospitals
- ^ The Disappearing Male – Sunday February 14, 2010 at 3 pm on CBC-TV, CBC
- ^ a b Pelley, Janet (November 2008). "Plasticizer may make boys less masculine". Environ Sci Technol. doi:10.1021/on.2008.11.12.154968. http://pubs.acs.org/action/showStoryContent?doi=10.1021%2Fon.2008.11.12.154968. Retrieved 2009-01-19.
- ^ Emily Main. "Fat's Hidden Trigger". Archived from the original on 20080829. http://web.archive.org/web/20080829164208/http://www.thegreenguide.com/doc/121/fat.
- ^ Pubmed
- ^ Gillum N, Karabekian Z, Swift LM, Brown RP, Kay MW, Sarvazyan N. Clinically relevant concentrations of Di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicology and Applied Pharmacology 2009, 236(1):25-38.
- ^ "《消費者報導雜誌》342期 第4至11頁「跟著流行走?踩著危機走!園丁鞋逾4成可塑劑超量」" (in zh-tw). Consumers' Foundation, Chinese Taipei (CFCT). http://www.consumers.org.tw/unit412.aspx?id=1245.
- ^ FOOD SCARE WIDENS:Tainted additives used for two decades: manufacturer, Taipei Times, May 29, 2011
- ^ 生活中心綜合報導 (2011-05-23). "塑化劑危機!台灣海洋深層水等4廠商飲料緊急下架" (in zh-tw). NOWnews. http://www.nownews.com/2011/05/23/327-2714830.htm. Retrieved 2011-5-25查閱.
External links
- FDA Public Health Notification: PVC devices containing the plasticizer DEHP
- ATSDR ToxFAQs
- National Pollutant Inventory - Bis 2 ethylhexyl phthalate fact sheet
- Eco-USA
- DEHP Information Centre (pro-DEHP, industry-backed group)
- Report on DEHP by noharm.org
- Industry report
- Detailed overview of medical DEPH effects and strategies to avoid it (German)
- Spectrum Laboratories Fact Sheet
- ChemSub Online : Bis(2-ethylhexyl) phthalate -DEHP.
Health issues of plastics and Polyhalogenated compounds (PHCs) Plasticizers: Phthalates Miscellaneous plasticizers Monomers Bisphenol A (BPA, in Polycarbonates) · Vinyl chloride (in PVC)Miscellaneous additives incl. PHCs Health issues Miscellanea PVC · Plastic recycling · Plastic bottle · Vinyl chloride · Dioxins · Polystyrene · Styrofoam · PTFE (Teflon) · California Proposition 65 · List of environmental health hazards · Persistent organic pollutant · European REACH regulation · Japan Toxic Substances Law · Toxic Substances Control ActCategories:- Ester solvents
- Phthalates
- Endocrine disruptors
- Plasticizers
- O=C(OCC(CC)CCCC)
Wikimedia Foundation. 2010.
