- Polystyrene
-
For other uses, see Polystyrene (disambiguation).
Polystyrene (
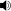 /ˌpɒliˈstaɪriːn/; IUPAC poly(1-phenylethene-1,2-diyl)) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry. Polystyrene is one of the most widely used plastics, the scale being several billion kilograms per year.
/ˌpɒliˈstaɪriːn/; IUPAC poly(1-phenylethene-1,2-diyl)) also known as Thermocole, abbreviated following ISO Standard PS, is an aromatic polymer made from the monomer styrene, a liquid hydrocarbon that is manufactured from petroleum by the chemical industry. Polystyrene is one of the most widely used plastics, the scale being several billion kilograms per year.Polystyrene is a thermoplastic substance, which is in solid (glassy) state at room temperature, but flows if heated above its glass transition temperature of about 100 °C (for molding or extrusion), and becomes solid again when cooled. Pure solid polystyrene is a colorless, hard plastic with limited flexibility. It can be cast into molds with fine detail. Polystyrene can be transparent or can be made to take on various colors.
Solid polystyrene is used, for example, in disposable cutlery, plastic models, CD and DVD cases, and smoke detector housings. Products made from foamed polystyrene are nearly ubiquitous, for example packing materials, insulation, and foam drink cups.
Polystyrene can be recycled, and has the number "6" as its recycling symbol. The increasing oil prices have increased the value of polystyrene for recycling. No known microorganism has yet been shown to biodegrade polystyrene, and it is often abundant as a form of pollution in the outdoor environment, particularly along shores and waterways especially in its low density cellular form.
Contents
History
Polystyrene was discovered in 1839 by Eduard Simon,[1] an apothecary in Berlin. From storax, the resin of the Turkish sweetgum tree Liquidambar orientalis, he distilled an oily substance, a monomer that he named styrol. Several days later, Simon found that the styrol had thickened, presumably from oxidation, into a jelly he dubbed styrol oxide ("Styroloxyd"). By 1845 English chemist John Blyth and German chemist August Wilhelm von Hofmann showed that the same transformation of styrol took place in the absence of oxygen. They called their substance metastyrol. Analysis later showed that it was chemically identical to Styroloxyd. In 1866 Marcelin Berthelot correctly identified the formation of metastyrol from styrol as a polymerization process. About 80 years went by before it was realized that heating of styrol starts a chain reaction that produces macromolecules, following the thesis of German organic chemist Hermann Staudinger (1881–1965). This eventually led to the substance receiving its present name, polystyrene.
The company I. G. Farben began manufacturing polystyrene in Ludwigshafen, Germany, about 1931, hoping it would be a suitable replacement for die-cast zinc in many applications. Success was achieved when they developed a reactor vessel that extruded polystyrene through a heated tube and cutter, producing polystyrene in pellet form.
Before 1949, the chemical engineer Fritz Stastny (1908–1985) developed pre-expanded PS beads by incorporating aliphatic hydrocarbons, such as pentane. These beads are the raw material for moulding parts or extruding sheets. BASF and Stastny applied for a patent that was issued in 1949. The moulding process was demonstrated at the Kunststoff Messe 1952 in Düsseldorf. Products were named Styropor.
The crystal structure of isotactice polystyrene was reported by Giulio Natta.[2]
In 1959, the Koppers Company in Pittsburgh, Pennsylvania, developed expanded polystyrene (EPS) foam.[citation needed]
Structure
In chemical terms, polystyrene is a long chain hydrocarbon wherein alternating carbon centers are attached to phenyl groups (the name given to the aromatic ring benzene. Polystyrene's chemical formula is (C8H8)n; it contains the chemical elements carbon and hydrogen.
The materials properties are determined by short range van der Waals attractions between polymers chains. Since the molecules are long hydrocarbon chains that consist of thousands of atoms, the total attractive force between the molecules is large. When heated (or deformed at a rapid rate, due to a combination of viscoelastic and thermal insulation properties), the chains are able to take on a higher degree of conformation and slide past each other. This intermolecular weakness (versus the high intramolecular strength due to the hydrocarbon backbone) confers flexibility and elasticity. The ability of the system to be readily deformed above its glass transition temperature allows polystyrene (and thermoplastic polymers in general) to be readily softened and molded upon heating.
Polymerization
Polystyrene results when styrene monomers interconnect. In the polymerization, one carbon-carbon double bond (in the vinyl group) is replaced by a much stronger carbon-carbon single bond, hence it is very difficult to depolymerize polystyrene. About a few thousand monomers typically comprise a chain of polystyrene, giving a molecular weight of 100,000–400,000.
A 3-D model would show that each of the chiral backbone carbons lies at the center of a tetrahedron, with its 4 bonds pointing toward the vertices. Let's consider that the -C-C- bonds are rotated so that the backbone chain lies entirely in the plane of the diagram. From this flat schematic, it is not evident which of the phenyl (benzene) groups are angled toward us from the plane of the diagram, and which ones are angled away. The isomer where all of them are on the same side is called isotactic polystyrene, which is not produced commercially.
Atactic polystyrene
The only commercially important form of polystyrene is atactic, which means that the phenyl groups are randomly distributed on both sides of the polymer chain. This random positioning prevents the chains from ever aligning with sufficient regularity to achieve any crystallinity. The plastic has a glass transition temperature Tg of ~90C. Polymerization is initiated with free radicals.[3]
Isotactic and syndiotactic polystyrene
Ziegler-Natta polymerization can produce an ordered syndiotactic polystyrene with the phenyl groups positioned on alternating sides of the hydrocarbon backbone. This form is highly crystalline with a Tm of 270 °C (518 °F). Such materials are not commercially produced because the polymerization is slow.
Extruded polystyrene is about as strong as an unalloyed aluminium, but much more flexible and much lighter (1.05 g/cm3 vs. 2.70 g/cm3 for aluminium).
Degradation
Because it is an aromatic hydrocarbon, it burns with an orange-yellow flame, giving off soot, as is characteristic of materials containing aromatic rings. Complete oxidation of polystyrene produces carbon dioxide and water vapor. Because of its chemical inertness, polystyrene is used to fabricate containers for chemicals, solvents, and foods. Polystyrene contains traces of styrene monomer. When food is heated in polystyrene container the monomer is extracted and enter the digestive system of the consumer. Styrene is toxic and a known carcinogen. This causes additional concerns when used for food or beverage packaging. Polystyrene is soluble in most of the organic solvents known and is not appropriate for such uses. Foamed polystyrene is used for packaging purposes of chemicals, but it does not come into contact with the actual solvents.
Forms produced
Properties Density 1.05 g/cm3 Density of EPS 16–640 kg/m3[4] Dielectric constant 2.4–2.7 Electrical conductivity (s) 10−16 S/m Thermal conductivity (k) 0.08 W/(m·K) Young's modulus (E) 3000–3600 MPa Tensile strength (st) 46–60 MPa Elongation at break 3–4% Notch test 2–5 kJ/m2 Glass transition temperature 95 °C Melting point[5] 240 °C Vicat B 90 °C[6] Linear expansion coefficient (a) 8×10−5 /K Specific heat (c) 1.3 kJ/(kg·K) Water absorption (ASTM) 0.03–0.1 Decomposition X years, still decaying Polystyrene is commonly injection molded or extruded, while expanded polystyrene is either extruded or molded in a special process. Polystyrene copolymers are also produced; these contain one or more other monomers in addition to styrene. In recent years the expanded polystyrene composites with cellulose[7][8] and starch[9] have also been produced.
Extruded closed-cell polystyrene foam is sold under the trademark Styrofoam by Dow Chemical. This term is often used informally for other foamed polystyrene products.
Polystyrene is used in some polymer-bonded explosives:
Polystyrene PBX examples Name Explosive ingredients Binder ingredients PBX-9205 RDX 92% Polystyrene 6%; DOP 2% PBX-9007 RDX 90% Polystyrene 9.1%; DOP 0.5%; resin 0.4% Sheet or molded polystyrene
Polystyrene (PS) is economical, and is used for producing plastic model assembly kits, plastic cutlery, CD "jewel" cases, smoke detector housings, license plate frames, and many other objects where a fairly rigid, economical plastic is desired. Production methods include thermoforming and injection molding.
Polystyrene Petri dishes and other laboratory containers such as test tubes and microplates play an important role in biomedical research and science. For these uses, articles are almost always made by injection molding, and often sterilized post-molding, either by irradiation or by treatment with ethylene oxide. Post-mold surface modification, usually with oxygen-rich plasmas, is often done to introduce polar groups. Much of modern biomedical research relies on the use of such products; they therefore play a critical role in pharmaceutical research.[10]
Foams
Polystyrene foams are good thermal insulators and are therefore often used as building insulation materials, such as in structural insulated panel building systems. They are also used for non-weight-bearing architectural structures (such as ornamental pillars). PS foams exhibit also good damping properties, therefore it is used widely in packaging.
Expanded polystyrene
Expanded polystyrene (EPS) is a rigid and tough, closed-cell foam. It is usually white and made of pre-expanded polystyrene beads. Familiar uses include molded sheets for building insulation and packing material ("peanuts") for cushioning fragile items inside boxes. Sheets are commonly packaged as rigid panels (size 4 by 8 or 2 by 8 feet in the United States), which are also known as "bead-board". Thermal resistivity is usually about 28 m·K/W (or R-4 per inch). Some EPS boards have a flame spread of less than 25 and a smoke-developed index of less than 450, which means they can be used without a fire barrier (but require a 15-minute thermal barrier) according to US building codes. A growing use of EPS in construction is insulating concrete forms. The density range is about 16–640 kg/m3.[4] The most common processing method is thermal cutting with hot wires.[11]
Extruded polystyrene foam
Extruded polystyrene foam (XPS) consists of closed cells, offers improved surface roughness and higher stiffness and reduced thermal conductivity. The density range is about 28 – 45 kg/m3.
Extruded polystyrene material is also used in crafts and model building, in particular architectural models. Because of the extrusion manufacturing process, XPS does not require facers to maintain its thermal or physical property performance. Thus, it makes a more uniform substitute for corrugated cardboard. Thermal resistivity is usually about 35 m·K/W (or R-5 per inch in American customary units).
Styrofoam is a trademarked name for XPS; however, it is often also used in the United States as a generic name for all polystyrene foams.
Copolymers
Pure polystyrene is brittle, but hard enough that a fairly high-performance product can be made by giving it some of the properties of a stretchier material, such as polybutadiene rubber. The two such materials can never normally be mixed because of the amplified effect of intermolecular forces on polymer insolubility (see plastic recycling), but if polybutadiene is added during polymerization it can become chemically bonded to the polystyrene, forming a graft copolymer, which helps to incorporate normal polybutadiene into the final mix, resulting in high-impact polystyrene or HIPS, often called "high-impact plastic" in advertisements. One commercial name for HIPS is Bextrene. Common applications of HIPS include toys and product casings. HIPS is usually injection molded in production. Autoclaving polystyrene can compress and harden the material.
Several other copolymers are also used with styrene. Acrylonitrile butadiene styrene or ABS plastic is similar to HIPS: a copolymer of acrylonitrile and styrene, toughened with polybutadiene. Most electronics cases are made of this form of polystyrene, as are many sewer pipes. SAN is a copolymer of styrene with acrylonitrile, and SMA one with maleic anhydride. Styrene can be copolymerized with other monomers; for example, divinylbenzene can be used for cross-linking the polystyrene chains to give the polymer used in Solid phase peptide synthesis.
Oriented polystyrene
Oriented polystyrene (OPS) is produced by stretching extruded PS film, improving visibility through the material by reducing haziness and increasing stiffness. This is often used in packaging where the manufacturer would like the consumer to see the enclosed product. Some benefits to OPS are that it is less expensive to produce than other clear plastics such as PP, PET, and HIPS, and it is less hazy than HIPS or PP. The main disadvantage to OPS is that it's brittle. It will crack or tear easily.
Disposal and environmental issues
Polystyrene is easily recycled. Due its light weight (especially if foamed), it is not economical to collect in its original form. However, if the waste material goes through an initial compaction process, the material changes density from typically 30 kg/m3 to 330 kg/m3 and becomes a recyclable commodity of high value for producers of recycled plastic pellets. In general, it is not accepted in curbside collection recycling programs. In Germany, polystyrene is collected, as a consequence of the packaging law (Verpackungsverordnung) that requires manufacturers to take responsibility for recycling or disposing of any packaging material they sell. In the US and many other countries, the interest in recycling polystyrene has led to the establishment of collection points. The producers of large quantities of polystyrene waste (50 tons per year or more) that have invested in the EPS compactors are able to sell the compacted blocks to plastic recyclers.
Environmental impact
Discarded polystyrene does not biodegrade for hundreds of years and is resistant to photolysis.[12] Because of this stability, very little of the waste discarded in today's modern, highly engineered landfills biodegrades. Because degradation of materials creates potentially harmful liquid and gaseous by-products that could contaminate groundwater and air, today's landfills are designed to minimize contact with air and water required for degradation, thereby practically eliminating the degradation of waste.[13]
Polystyrene foam is a major component of plastic debris in the ocean, where it becomes toxic to marine life. Foamed polystyrene blows in the wind and floats on water, and is abundant in the outdoor environment. Polystyrene foams are produced using blowing agents that form bubbles and expand the foam. In expanded polystyrene, these are usually hydrocarbons such as pentane, which may pose a flammability hazard in manufacturing or storage of newly manufactured material, but have relatively mild environmental impact. However, extruded polystyrene is usually made with hydrochlorofluorocarbons (HCFC-22)[14], which have a 1000-times greater 'greenhouse effect' on global warming compared to carbon dioxide.[15]
Recycling
Most polystyrene products are currently not recycled due to the lack of incentive to invest in the compactors and logistical systems required. Expanded polystyrene scrap can be easily added to products such as EPS insulation sheets and other EPS materials for construction applications. And many manufacturers cannot obtain sufficient scrap because of the aforementioned collection issues. When it is not used to make more EPS, foam scrap can be turned into clothes hangers, park benches, flower pots, toys, rulers, stapler bodies, seedling containers, picture frames, and architectural molding from recycled PS.[16]
Recycled EPS is also used in many metal casting operations. Rastra is made from EPS that is combined with cement to be used as an insulating amendment in the making of concrete foundations and walls. American manufacturers have produced insulating concrete forms made with approximately 80% recycled EPS since 1993. However, polystyrene recycling is not a closed loop, producing more polystyrene; polystyrene cups and other packaging materials are instead usually used as fillers in other plastics, or in other items that cannot themselves be recycled and are thrown away.[citation needed]
Incineration
If polystyrene is properly incinerated at high temperatures, the chemicals generated are water, carbon dioxide, a complex mixture of volatile compounds, and carbon soot.[17] According to the American Chemistry Council, when polystyrene is incinerated in modern facilities, the final volume is 1% of the starting volume; most of the polystyrene is converted into carbon dioxide, water vapor, and heat. Because of the amount of heat released, it is sometimes used as a power source for steam or electricity generation.[18]
When polystyrene was burned at temperatures of 800–900 °C (the typical range of a modern incinerator), the products of combustion consisted of "a complex mixture of polycyclic aromatic hydrocarbons (PAHs) from alkyl benzenes to benzoperylene. Over 90 different compounds were identified in combustion effluents from polystyrene."[19]
When burned without enough oxygen or at lower temperatures (as in a campfire or a household fireplace), polystyrene can produce polycyclic aromatic hydrocarbons, carbon black, and carbon monoxide, as well as styrene monomers.[17][20]
Burial
Foam cups and other polystyrene products can be safely buried[citation needed] in landfills, since it is as stable as concrete or brick. No plastic film is required to protect the air and underground water[citation needed].
Reducing
Some effort is being made to find alternatives to polystyrene foam, especially in restaurant settings. Restricting the use of foamed polystyrene takeout food packaging is a priority of many solid waste environmental organizations. However, the Plastics Foodservice Packaging Group counters that, in US, less than 1% by weight of solid waste disposed is polystyrene[citation needed]. A campaign to achieve the first ban of polystyrene foam from the food & beverage industry in Canada was launched in Toronto as of January 2007, by local non-profit organization NaturoPack.[21] Portland, Ore. and San Francisco are among about one hundred cities in the United States that currently have some sort of ban on polystyrene foam in restaurants. For instance, in 2007 restaurants in Oakland, California were required to switch to disposable food containers that will biodegrade if added to food compost.[22]
Although polystyrene can be recycled at recycling facilities, most polystyrene is not recycled. The EPA (United States Environmental Protection Agency) estimates that 25 billion polystyrene cups are tossed every year. Since polystyrene degrades very slowly - more than 500 years for a single cup[citation needed] – the EPA considers this a serious environmental problem. Several green leaders, from the Dutch Ministry of the Environment to Starbucks' Green Team, advise that individuals reduce their environmental impact by using reusable coffee cups.[23]
Finishing
In the United States, environmental protection regulations prohibit the use of solvents on polystyrene (which would dissolve the polystyrene and de-foam most foams anyway).
Some acceptable finishing materials are
- Water-based paint (artists have created paintings on polystyrene with gouache)
- Mortar or acrylic/cement render, often used in the building industry as a weather-hard overcoat that hides the foam completely after finishing the objects.
- Cotton wool or other fabrics used in conjunction with a stapling implement.
Safety
Health
According to a plastic food service products website:
Based on scientific tests over five decades, government safety agencies have determined that polystyrene is safe for use in foodservice products. For example, polystyrene meets the stringent standards of the U.S. Food and Drug Administration and the European Commission/European Food Safety Authority for use in packaging to store and serve food. The Hong Kong Food and Environmental Hygiene Department recently reviewed the safety of serving various foods in polystyrene foodservice products and reached the same conclusion as the U.S. FDA.[24]From 1999 to 2002, a comprehensive review of the potential health risks associated with exposure to styrene was conducted by a 12 member international expert panel selected by the Harvard Center for Risk Assessment. The scientists had expertise in toxicology, epidemiology, medicine, risk analysis, pharmacokinetics, and exposure assessment.
The Harvard study reported that styrene is naturally present in foods such as strawberries, beef, and spices, and is naturally produced in the processing of foods such as wine and cheese. The study also reviewed all the published data on the quantity of styrene contributing to the diet due to migration of food packaging and disposable food contact articles, and concluded there is no cause for concern for the general public from exposure to styrene from foods or styrenic materials used in food-contact applications, such as polystyrene packaging and food service containers.[25]Styrene oligomers in polystyrene containers used for food packaging have been found to migrate into the food.[26] Another Japanese study conducted on wild-type and AhR-null mice found that the styrene trimer, which the authors detected in cooked polystyrene container-packed instant foods, may increase thyroid hormone levels.[27]
Fire hazards
Like other organic compounds, polystyrene is flammable. Polystyrene is classified according to DIN4102 as a "B3" product, meaning highly flammable or "easily ignited."[citation needed] As a consequence, although it is an efficient insulator at low temperatures, its use is prohibited in any exposed installations in building construction if the material is not flame-retardant.[citation needed] It must be concealed behind drywall, sheet metal, or concrete.[citation needed] Foamed polystyrene plastic materials have been accidentally ignited and caused huge fires and losses, for example at the Düsseldorf International Airport, the Channel tunnel (where polystyrene was inside a railcar that caught fire), and the Browns Ferry Nuclear Power Plant (where fire breached a fire retardant and reached the foamed plastic underneath, inside a firestop that had not been tested and certified in accordance with the final installation).[citation needed]
See also
References
- ^ The history of plastics
- ^ G. Natta, P. Corradini, I.W. Bassi (1960). "Crystal structure of isotactic polystyrene". Il Nuovo Cimento 15: 68–82. doi:10.1007/BF02731861.
- ^ J. Maul, B. G. Frushour, J. R. Kontoff, H. Eichenauer, K.-H. Ott, C. Schade Polystyrene and Styrene Copolymers" in Ullmann's Encyclopedia of Industrial Chemistry 2007 Wiley-VCH, Weinheim.doi:10.1002/14356007.a21_615.pub2
- ^ a b K. Goodier (June 22, 1961). "Making and using an expanded plastic". New Scientist 240: 706. http://books.google.com/?id=d_XOKdeyXrYC&pg=PA706.
- ^ International Labour Organisation chemical safety card for polystyrene
- ^ A.K. van der Vegt & L.E. Govaert, Polymeren, van keten tot kunstof, ISBN 90-407-2388-5
- ^ Doroudiani S, Kortschot MT (2004). "Expanded Wood Fiber Polystyrene Composites: Processing-Structure-Mechanical Properties Relationships". Journal of Thermoplastic Composite Materials 17: 13–30. doi:10.1177/0892705704035405.
- ^ Doroudiani, Saeed; Chaffey, Charles E.; Kortschot, Mark T. (2002). "Sorption and diffusion of carbon dioxide in wood-fiber/polystyrene composites". Journal of Polymer Science Part B: Polymer Physics 40 (8): 723. doi:10.1002/polb.10129.
- ^ Mihai, M.; Huneault, M. A.; Favis, B. D. (2007). "Foaming of Polystyrene/ Thermoplastic Starch Blends". Journal of Cellular Plastics 43 (3): 215. doi:10.1177/0021955X07076532.
- ^ Jed Norton. "Blue Foam, Pink Foam and Foam Board". Antenociti's Workshop. Archived from the original on 2008-02-26. http://web.archive.org/web/20080226152632/http://barrule.com/workshop/images/info/foams/index.htm. Retrieved 2008-01-29.
- ^ Expandable Polystyrene, Insight database from Ceresana Research
- ^ Bandyopadhyay, Abhijit; Chandra Basak, G. (2007). "Studies on photocatalytic degradation of polystyrene". Materials Science and Technology 23 (3): 307–317. doi:10.1179/174328407X158640.
- ^ William Rathje and Cullen Murphy (1989). Rubbish! The Archeology of Garbage.
- ^ [1]:Earth Resource Foundation.
- ^ IPCC Third Assessment Report, Climate Change 2001: Working Group I: The Scientific Basis. Section 6.12.2 Direct GWPs.
- ^ Polystyrene recycling. Polystyrene packaging council. Retrieved on 2009-03-06.
- ^ a b Polystyrene Foam Burning Danger
- ^ "Ease of Disposal". http://www.americanchemistry.com/s_plastics/sec_pfpg.asp?CID=1434&did=5226. Retrieved 2009-06-25.
- ^ Hawley-Fedder, R.A.; Parsons, M.L. and Karasek, F.W. (1984). "Products obtained during combustion of polymers under simulated incinerator conditions II. Polystyrene". Products Obtained During Combustion of Polymers Under Simulated Incinerator Conditions, II Polystyrene 315: 201. doi:10.1016/S0021-9673(01)90737-X. http://www.ejnet.org/plastics/polystyrene/disposal.html.
- ^ Burning Polystyrene Foam
- ^ Naturopack Campaign Page
- ^ Hadish, Cindy. "Food for thought: 100 U.S. cities enact bans." Gazette, The (Cedar Rapids, IA) 2 Apr. 2008.
- ^ Dineen, Shauna (Nov. – Dec. 2005). "The Throwaway Generation: 25 Billion Styrofoam Cups a Year". E-The Environmental Magazine. http://www.emagazine.com/view/?2933.
- ^ Staff (2010-2011). "Q & A on the Safety of Polystyrene Foodservice Products". American Chemistry Council. http://plasticfoodservicefacts.com/main/Safety/Californias-Proposition-65/Q-A-on-the-Safety-of-Polystyrene-Foodservice-Products.GMEditor.html. Retrieved 2011-06-14.
- ^ Cohen, Joshua T.; Carlson, Gary; Charnley, Gail; Coggon, David; Delzell, Elizabeth; Graham, John D.; Greim, Helmut; Krewski, Daniel et al. (2002). "A comprehensive evaluation of the potential health risks associated with occupational and environmental exposure to styrene". Journal of Toxicology and Environmental Health Part B: Critical Reviews 5: 1. doi:10.1080/10937400252972162.
- ^ Sakamato H, Matsuzawa A, Itoh R, Tohyama Y (2000). "Quantitative Analysis of Styrene Dimer and Trimers Migrated from Disposable Lunch Boxes". J Food Hyg Soc Japan 41 (3): 200–205. doi:10.3358/shokueishi.41.200. http://sciencelinks.jp/j-east/article/200016/000020001600A0689499.php.
- ^ Yanagiba, Yukie et al. (2008). "Styrene Trimer May Increase Thyroid Hormone Levels via Down-Regulation of the Aryl Hydrocarbon Receptor (AhR) Target Gene UDP-Glucuronosyltransferase" (free text). Environmental Health Perspectives 116 (6): 740–745. doi:10.1289/ehp.10724. PMC 2430229. PMID 18560529. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2430229.
External links
- Polystyrene Composition – The University of Southern Mississippi
- SPI resin identification code – Society of the Plastics Industry
- Bacteria Turns Styrofoam into Biodegradable Plastic – Scientific American, February 27, 2006
- Polystyrene (packaging) facts
Health issues of plastics and Polyhalogenated compounds (PHCs) Plasticizers: Phthalates Miscellaneous plasticizers Monomers Bisphenol A (BPA, in Polycarbonates) · Vinyl chloride (in PVC)Miscellaneous additives incl. PHCs Health issues Miscellanea PVC · Plastic recycling · Plastic bottle · Vinyl chloride · Dioxins · Polystyrene · Styrofoam · PTFE (Teflon) · California Proposition 65 · List of environmental health hazards · Persistent organic pollutant · European REACH regulation · Japan Toxic Substances Law · Toxic Substances Control ActPlastics Polyacrylic acid (PAA) · Cross-linked polyethylene (PEX, XLPE) · Polyethylene (PE) · Polyethylene terephthalate (PET, PETE) · Polyphenyl ether (PPE) · Polyvinyl chloride (PVC) · Polyvinylidene chloride (PVDC) · Polylactic acid (PLA) · Polypropylene (PP) · Polybutylene (PB) · Polybutylene terephthalate (PBT) · Polyamide (PA) · Polyimide (PI) · Polycarbonate (PC) · Polytetrafluoroethylene (PTFE) · Polystyrene (PS) · Polyurethane (PU) · Polyester (PEs) · Acrylonitrile butadiene styrene (ABS) · Poly(methyl methacrylate) (PMMA) · Polyoxymethylene (POM) · Polysulfone (PES) · Styrene-acrylonitrile (SAN) · Ethylene vinyl acetate (EVA) · Styrene maleic anhydride (SMA)
Categories:- Insulators
- Building insulation materials
- Organic polymers
- Packaging materials
- Thermoplastics
Wikimedia Foundation. 2010.





