- Maleic anhydride
-
Maleic anhydride[1] 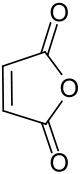
 Furan-2,5-dione
Furan-2,5-dioneIdentifiers CAS number 108-31-6 
PubChem 7923 ChemSpider 7635 
UNII V5877ZJZ25 
EC-number 203-571-6 ChEBI CHEBI:474859 
ChEMBL CHEMBL374159 
RTECS number UE5950000 Jmol-3D images Image 1 - C1=CC(=O)OC1=O
Properties Molecular formula C4H2O3 Molar mass 98.06 g/mol Appearance White crystals Density 1.48 g/cm3 Melting point 52.8 °C, 326 K, 127 °F
Boiling point 202 °C, 475 K, 396 °F
Solubility in water Reacts Hazards MSDS MSDS at J. T. Baker EU classification Corrosive (C) R-phrases R22, R34, R42/43 S-phrases (S2), S22, S26,
S36/37/39, S45NFPA 704 Flash point 102 °C Related compounds Related acid anhydrides Succinic anhydride Related compounds Maleic acid  anhydride (verify) (what is:
anhydride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Maleic anhydride (cis-butenedioic anhydride, toxilic anhydride, 2,5-dioxofuran) is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour.
Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene; due to rising benzene prices, most maleic anhydride plants now use n-butane as a feedstock:
- 2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O
Reactions
The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity. It hydrolyzes, producing maleic acid, cis-HOOC–CH=CH–COOH. With alcohols, the half-ester is generated, e.g., cis-HOOC–CH=CH–COOCH3.
Maleic anhydride is a potent dienophile in Diels-Alder reactions. It is also a ligand for low-valent metal complexes, examples being Pt(PPh3)2(MA) and Fe(CO)4(MA).
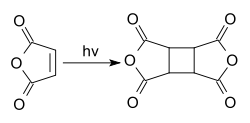
Maleic anhydride dimerizes in a photochemical reaction to form cyclobutane tetracarboxylic dianhydride (CBTA). This compound is used in the production of polyimides and as an alignment film for liquid crystal displays.[2][3]
References
- ^ Merck Index, 11th Edition, 5586.
- ^ Horie, T.; Sumino, M.; Tanaka, T.; Matsushita, Y.; Ichimura, T.; Yoshida, J. I. (2010). "Photodimerization of Maleic Anhydride in a Microreactor Without Clogging". Organic Process Research & Development 14 (2): 100128104701019. doi:10.1021/op900306z.
- ^ Reaction conditions Horie et al 2010 reaction conditions: microreactor , mercury lamp, ethyl acetate solvent, 15 °C
External links
Categories:- Acid anhydrides
- Alkenes
- 2,5-Dihydrofurans
Wikimedia Foundation. 2010.