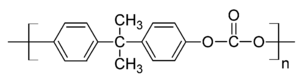
- Polycarbonate
-
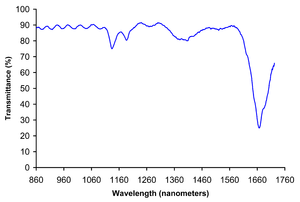
Polycarbonate Physical Properties Density (ρ) 1.20–1.22 g/cm3 Abbe number (V) 34.0 Refractive index (n) 1.584–1.586 Flammability V0-V2 Limiting oxygen index 25–27% Water absorption – Equilibrium(ASTM) 0.16–0.35% Water absorption – over 24 hours 0.1% Radiation resistance Fair Ultraviolet (1-380nm) resistance Fair Mechanical Properties Young's modulus (E) 2.0–2.4 GPa Tensile strength (σt) 55–75 MPa Compressive strength (σc) >80 MPa Elongation (ε) @ break 80–150% Poisson's ratio (ν) 0.37 Hardness – Rockwell M70 Izod impact strength 600–850 J/m Notch test 20–35 kJ/m2 Abrasive resistance – ASTM D1044 10–15 mg/1000 cycles Coefficient of friction (μ) 0.31 Speed of sound 2270 m/s Thermal Properties Melting temperature (Tm) 267 °C* Glass transition temperature(Tg) 150 °C Heat deflection temperature – 10 kN (Vicat B)[citation needed] 145 °C Heat deflection temperature – 0.45 MPa 140 °C Heat deflection temperature – 1.8 MPa 128–138 °C Upper working temperature 115–130 °C Lower working temperature −40 °C[1] Linear thermal expansion coefficient (α) 65–70 × 10−6/K Specific heat capacity (c) 1.2–1.3 kJ/(kg·K) Thermal conductivity (k) @ 23 °C 0.19–0.22 W/(m·K) Electrical Properties Dielectric constant (εr) @ 1 MHz 2.9 Permittivity (ε) @ 1 MHz 2.568 × 10−11 F/m Relative permeability (μr) @ 1 MHz 0.866(2) Permeability (μ) @ 1 MHz 1.089(2) μN/A2 Dielectric strength 15–67 kV/mm Dissipation factor @ 1 MHz 0.01 Surface resistivity 1015 Ω/sq Volume resistivity (ρ) 1012–1014 Ω·m Near to Short-wave Infrared Transmittance Spectrum Chemical Resistance Acids – concentrated Poor Acids – dilute Good Alcohols Good Alkalis Good-Poor Aromatic hydrocarbons Poor Greases & Oils Good-fair Halogenated Hydrocarbons Good-poor Halogens Poor Ketones Poor Gas permeation @ 20 °C Nitrogen 10 – 25 cm3·mm/(m2·day·Bar) Oxygen 70 – 130 cm3·mm/(m2·day·Bar) Carbon dioxide 400 – 800 cm3·mm/(m2·day·Bar) Water vapour 1–2 gram·mm/(m2·day) @ 85%–0% RH gradient) Economic Properties Price 2.6 – 2.8 €/kg[2] Polycarbonates, known by the trademarked names Lexan, Makrolon, Makroclear and others, are a particular group of thermoplastic polymers. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique plastic identification code and are identified as Other, 7.
Contents
Structure
Polycarbonates received their name because they are polymers containing carbonate groups (–O–(C=O)–O–). Most polycarbonates of commercial interest are derived from rigid monomers. A balance of useful features including temperature resistance, impact resistance and optical properties position polycarbonates between commodity plastics and engineering plastics.
Production
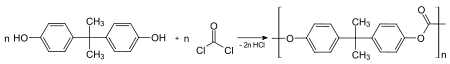
The main polycarbonate material is produced by the reaction of bisphenol A and phosgene COCl2. The overall reaction can be written as follows:
The first step of the synthesis involves treatment of bisphenol A with sodium hydroxide, which deprotonates the hydroxyl groups of the bisphenol A.[3]
- (HOC6H4)2CMe2 + 2 NaOH → (NaOC6H4)2CMe2 + 2 H2O
The diphenoxide ((NaOC6H4)2CMe2) reacts with phosgene to give a chloroformate, which subsequently is attacked by another phenoxide. The net reaction from the diphenoxide is:
- (NaOC6H4)2CMe2 + COCl2 → 1/n [OC(OC6H4)2CMe2]n + 2 NaCl
In this way, approximately one billion kilograms of polycarbonate is produced annually. Many other diols have been tested in place of bisphenol A, e.g. 1,1-bis(4-hydroxyphenyl)cyclohexane and dihydroxybenzophenone including some, e.g. tetramethylcyclobutanediol, that are unlikely endocrine disruptors.
An alternative route to polycarbonates entails transesterification from BPA and diphenyl carbonate:
- (HOC6H4)2CMe2 + (C6H5O)2CO → 1/n [OC(OC6H4)2CMe2]n + 2 C6H5OH
The diphenyl carbonate was derived in part from carbon monoxide, this route being greener than the phosgene method.[3]
Properties and processing
Polycarbonate is a very durable material. Although it has high impact-resistance, it has low scratch-resistance and so a hard coating is applied to polycarbonate eyewear lenses and polycarbonate exterior automotive components. The characteristics of polycarbonate are quite like those of polymethyl methacrylate (PMMA, acrylic), but polycarbonate is stronger, usable in a wider temperature range but more expensive. This polymer is highly transparent to visible light and has better light transmission characteristics than many kinds of glass.
Polycarbonate has a glass transition temperature of about 150 °C (302 °F), so it softens gradually above this point and flows above about 300 °C (572 °F). Tools must be held at high temperatures, generally above 80 °C (176 °F) to make strain- and stress-free products. Low molecular mass grades are easier to mold than higher grades, but their strength is lower as a result. The toughest grades have the highest molecular mass, but are much more difficult to process.
Unlike most thermoplastics, polycarbonate can undergo large plastic deformations without cracking or breaking. As a result, it can be processed and formed at room temperature using sheet metal techniques, such as forming bends on a brake. Even for sharp angle bends with a tight radius, no heating is generally necessary. This makes it valuable in prototyping applications where transparent or electrically non-conductive parts are needed, which cannot be made from sheet metal. Note that PMMA/Plexiglas, which is similar in appearance to polycarbonate, is brittle and cannot be bent at room temperature.
Main transformation techniques for polycarbonate resins:
- extrusion into tubes, rods and other profiles
- extrusion with cylinders into sheets (0.5–15 mm (0.020–0.59 in)) and films (below 1 mm (0.039 in)), which can be used directly or manufactured into other shapes using thermoforming or secondary fabrication techniques, such as bending, drilling, routing, laser cutting etc.
- injection molding into ready articles
Applications
Electronic components
Polycarbonate is mainly used for electronic applications that capitalize on its collective safety features. Being a good electrical insulator and having heat resistant and flame retardant properties, it is used in various products associated with electrical and telecommunications hardware. It also serves as dielectric in high stability capacitors.[3]
Construction materials
The second largest consumer of polycarbonates is the construction industry, e.g. for domelights, flat or curved glazing, and sound walls.
Data storage
A major application of polycarbonate is the production of Compact Discs, DVDs, and Blu-ray Discs. These discs are produced by injection molding polycarbonate into a mold cavity that has on one side a metal stamper containing a negative image of the disc data, while the other mold side is a mirrored surface. Typical products of sheet/film production include applications in advertisement (signs, displays, poster protection).[3]
Automotive, aircraft, and security components
In the automotive industry, injection-molded polycarbonate can produce very smooth surfaces that make it well-suited for direct (without the need for a basecoat) metalised parts such as decorative bezels and optical reflectors. Its uniform mold shrinkage results in parts with greater accuracy than those made of polypropylene. However, due to its susceptibility to environmental stress cracking, its use is limited to low-stress applications. It can be laminated to make bullet-proof "glass", although "bullet-resistant" is more accurate for the thinner windows, such as are used in bullet-resistant windows in automobiles. The thicker barriers of transparent plastic used in teller's windows and barriers in banks are also polycarbonate.
So-called "theft-proof" large plastic packaging for smaller items, which cannot be opened by hand, is uniformly made from polycarbonate.
The cockpit canopy of the F-22 Raptor jet fighter is made from a piece of high optical quality polycarbonate, and is the largest piece of its type formed in the world.[4][5]
Niche applications
Polycarbonate, being a versatile material with attractive processing and physical properties, has attracted myriad smaller applications. The use of injection molded drinking bottles and glasses and food containers has stirred serious controversy (see Potential hazards in food contact applications).
Many kinds of lenses are manufactured from polycarbonate, automotive headlamp lenses, lighting lenses, sunglass/eyeglass, lenses, and safety glasses. Other miscellaneous items: including durable, lightweight luggage, MP3/digital audio player cases, ocarinas, computer cases, riot shields, visors, instrument panels, and blender jars. Many toys and hobby items are made from polycarbonate parts, e.g. fins, gyro mounts, and flybar locks for use with radio-controlled helicopters.[6]
For use in applications exposed to weathering or UV-radiation, a special surface treatment is needed. This either can be a coating (e.g. for improved abrasion resistance), or a coextrusion for enhanced weathering resistance.
Polycarbonate is also used as a printing substrate for nameplate and other forms of industrial grade under printed products. The polycarbonate provides a barrier to wear, the elements, and fading.
Medical applications
Some polycarbonate grades are used in medical applications and comply with both ISO 10993-1 and USP Class VI standards (occasionally referred to as PC-ISO). Class VI is the most stringent of the six USP ratings. These grades can be sterilized using steam at 120 °C, gamma radiation, or by the ethylene oxide (EtO) method.[7] However, scientific research indicates possible problems with biocompatibility. Dow Chemical strictly limits all its plastics with regard to medical applications.[8][9]
Potential hazards in food contact applications
The use of polycarbonate containers for the purpose of food storage is controversial. The basis of this controversy is their hydrolysis (degradation by water, often referred to as leaching) releases bisphenol A:
- 1/n [OC(OC6H4)2CMe2]n + H2O → (HOC6H4)2CMe2 + CO2
More than 100 studies have explored the bioactivity of bisphenol A derived from polycarbonates. Bisphenol A appeared to be released from polycarbonate animal cages into water at room temperature and it may have been responsible for enlargement of the reproductive organs of female mice.[10] However, the animal cages used in the research were fabricated from industrial grade polycarbonate, rather than FDA food grade polycarbonate.
An analysis of the literature on bisphenol A leachate low-dose effects by vom Saal and Hughes published in August 2005 seems to have found a suggestive correlation between the source of funding and the conclusion drawn. Industry funded studies tend to find no significant effects whereas government funded studies tend to find significant effects.[11]
Sodium hypochlorite bleach and other alkali cleaners catalyze the release of the bisphenol A from polycarbonate containers.[12][13] A chemical compatibility chart shows that polycarbonate is incompatible with ammonia and acetone because it dissolves in their presence.[14] Alcohol is one recommended organic solvent for cleaning grease and oils from polycarbonate.
See also
References
- ^ M. Parvin and J. G. Williams (1975). "The effect of temperature on the fracture of polycarbonate". Journal of Materials Science 10 (11): 1883. doi:10.1007/BF00754478.
- ^ CES Edupack 2010, Polycarbonate (PC) specs sheet
- ^ a b c d Volker Serini "Polycarbonates" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000. doi:10.1002/14356007.a21_207
- ^ Egress technicians keep raptor pilots covered. Pacaf.af.mil. Retrieved on 2011-02-26.
- ^ F-22 Cockpit. Globalsecurity.org (2008-01-21). Retrieved on 2011-02-26.
- ^ Hobby Applications of Polycarbonate
- ^ Medical Applications of Polycarbonate
- ^ "Dow Plastics Medical Application Policy". http://plastics.dow.com/plastics/medical/.
- ^ "Makrolon Polycarbonate Biocompatibility Grades". http://www.omnexus.com/tc/polycarbonate/index.aspx?id=biocompatibility.
- ^ Howdeshell, KL; Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, Vom Saal FS, Welshons WV (2003). "Bisphenol A is released from used polycarbonate animal cages into water at room temperature". Environmental Health Perspectives 111 (9): 1180–7. doi:10.1289/ehp.5993. PMC 1241572. PMID 12842771. http://ehp.niehs.nih.gov/members/2003/5993/5993.html. Retrieved 2006-06-07.
- ^ vom Saal FS, Hughes C (2005). "An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment". Environ. Health Perspect. 113 (8): 926–33. doi:10.1289/ehp.7713. PMC 1280330. PMID 16079060. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1280330.
- ^ Hunt, PA; Kara E. Koehler, Martha Susiarjo, Craig A. Hodges, Arlene Ilagan, Robert C. Voigt, Sally Thomas, Brian F. Thomas and Terry J. Hassold (2003). "Bisphenol A Exposure Causes Meiotic Aneuploidy in the Female Mouse". Current Biology 13 (7): 546–553. doi:10.1016/S0960-9822(03)00189-1. PMID 12676084.
- ^ Koehler, KE; Robert C. Voigt, Sally Thomas, Bruce Lamb, Cheryl Urban, Terry Hassold, and Patricia A. Hunt (2003). "When disaster strikes: rethinking caging materials". Lab Animal 32 (4): 24–27. doi:10.1038/laban0403-24. PMID 19753748. http://www.mindfully.org/Plastic/Plasticizers/BPA-Lab-Animal-CagesApr03.htm.
- ^ Premium Twinwall and Triplewall Polycarbonate Sheet by Verolite
Plastics Polyacrylic acid (PAA) · Cross-linked polyethylene (PEX, XLPE) · Polyethylene (PE) · Polyethylene terephthalate (PET, PETE) · Polyphenyl ether (PPE) · Polyvinyl chloride (PVC) · Polyvinylidene chloride (PVDC) · Polylactic acid (PLA) · Polypropylene (PP) · Polybutylene (PB) · Polybutylene terephthalate (PBT) · Polyamide (PA) · Polyimide (PI) · Polycarbonate (PC) · Polytetrafluoroethylene (PTFE) · Polystyrene (PS) · Polyurethane (PU) · Polyester (PEs) · Acrylonitrile butadiene styrene (ABS) · Poly(methyl methacrylate) (PMMA) · Polyoxymethylene (POM) · Polysulfone (PES) · Styrene-acrylonitrile (SAN) · Ethylene vinyl acetate (EVA) · Styrene maleic anhydride (SMA)
Health issues of plastics and Polyhalogenated compounds (PHCs) Plasticizers: Phthalates Miscellaneous plasticizers Monomers Bisphenol A (BPA, in Polycarbonates) · Vinyl chloride (in PVC)Miscellaneous additives incl. PHCs Health issues Miscellanea PVC · Plastic recycling · Plastic bottle · Vinyl chloride · Dioxins · Polystyrene · Styrofoam · PTFE (Teflon) · California Proposition 65 · List of environmental health hazards · Persistent organic pollutant · European REACH regulation · Japan Toxic Substances Law · Toxic Substances Control ActCategories:- Plastics
- Polymers
- Optical materials
- Polycarbonates
- Dielectrics
- Thermoplastics
- Transparent materials
Wikimedia Foundation. 2010.