- Acrylonitrile
-
Acrylonitrile 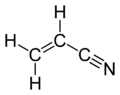
 2-propenenitrile
2-propenenitrileIdentifiers CAS number 107-13-1 
PubChem 7855 ChemSpider 7567 
UNII MP1U0D42PE 
KEGG C01998 
ChEBI CHEBI:28217 
ChEMBL CHEMBL445612 
Jmol-3D images Image 1
Image 2- N#CC=C
C=CC#N
Properties Molecular formula C3H3N Molar mass 53.06 g mol−1 Appearance Colourless liquid Density 0.81 g/cm3 Melting point -84 °C(189 K)
Boiling point 77 °C (350 K)
Solubility in water 7 g/100 mL at 20 °C Hazards MSDS ICSC 0092 Main hazards flammable,
reactive,
toxicNFPA 704 Related compounds Related compounds acrylic acid,
acrolein (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile. Pathways of exposure include emissions, auto exhaust, and cigarette smoke that can expose the human subject directly if they inhale or smoke. Routes of exposure include inhalation, oral, and occasional dermal routes from volunteer humans and rat studies.[1]
Contents
Uses
Acrylonitrile is used principally as a monomer to prepare the polyacrylonitrile, a homopolymer, or several important copolymers such as styrene-acrylonitrile (SAN), acrylonitrile butadiene styrene (ABS), acrylonitrile styrene acrylate (ASA) and other synthetic rubbers such as acrylonitrile butadiene (NBR). Dimerization of acrylonitrile affords adiponitrile, used in the synthesis of certain polyamides. Small amounts are also used as a fumigant. Acrylonitrile and derivatives such as 2-chloro-acrylonitrile are dienophiles in Diels-Alder reactions. Acrylonitrile is also a precursor in the industrial manufacture of acrylamide and acrylic acid.
Production
Of all the nitriles, Acrylonitrile is manufactured on probably the largest scale. Most industrial acrylonitrile is produced by catalytic ammoxidation of propene:
Health effects
Acrylonitrile is highly flammable and toxic. It undergoes explosive polymerization. The burning material releases fumes of hydrogen cyanide and oxides of nitrogen. The International Agency for Research on Cancer (IARC) concluded that there is inadequate evidence in humans for the carcinogenicity of acrylonitrile, but classified it as a Class 2B carcinogen (possibly carcinogenic). [2] Acrylonitrile increases cancer in high dose tests in male and female rats and mice. [3]
There are two main excretion processes of acrylonitrile. The primary method is excretion in urine when acrylonitrile is metabolized by being directly conjugated to glutathione. The other method is when acrylonitrile is metabolized with 2-cyanoethylene oxide to produce cyanide end products that ultimately forms thiocyanate, which is excreted via urine, or carbon dioxide and eliminated through the lungs. [1]
References
- ^ a b Acrylonitrile Fact Sheet: Support Document (CAS No. 107-13-1)
- ^ IARC evaluation of Acrylonitrile
- ^ Animal Test Result on Acrylonitrile in the Carcinogenic Potency Database
External links
Categories:- Nitriles
- Monomers
- Fumigants
- Hazardous air pollutants
- IARC Group 2B carcinogens
- Alkenes
- N#CC=C
Wikimedia Foundation. 2010.

