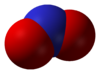
- Nitrogen dioxide
-
Nitrogen dioxide 
 Systematic nameDioxidonitrogen(•)[1] (additive)Other names
Systematic nameDioxidonitrogen(•)[1] (additive)Other namesIdentifiers CAS number 10102-44-0 
PubChem 3032552 ChemSpider 2297499 
EC number 233-272-6 UN number 1067 ChEBI CHEBI:33101 
RTECS number QW9800000 Gmelin Reference 976 Jmol-3D images Image 1
Image 2
Image 3- O=[N]=O
o:n:o
[O-][N++][O-]
Properties Molecular formula NO2• Molar mass 46.0055 g mol-1 Exact mass 45.992903249 g mol-1 Appearance Vivid orange gas Density 2.62 g dm-3 Boiling point 21 °C, 294 K, 70 °F
Solubility in water Reacts Vapor pressure 98.80 kPa (at 20 °C) Refractive index (nD) 1.449 (at 20 °C) Structure Molecular shape Dihedral digonal Hazards MSDS ICSC 0930 GHS pictograms 


GHS signal word Danger GHS hazard statements H270, H314, H330 GHS precautionary statements P220, P260, P280, P284, P305+351+338, P310 EU Index 007-002-00-0 EU classification  T+
T+R-phrases R26, R34, R8 S-phrases (S1/2), S9, S26, S28, S36/37/39, S45 NFPA 704 Related compounds Related Nitrogen oxides Dinitrogen pentoxide
Dinitrogen tetroxide
Dinitrogen trioxide
Nitric oxide
Nitrous oxide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitrogen dioxide is the chemical compound with the formula NO2 it is one of several nitrogen oxides. NO2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent air pollutant. Nitrogen dioxide is a paramagnetic bent molecule with C2v point group symmetry.
Contents
Molecular properties
Nitrogen dioxide has a molar mass of 46.0055, which makes it heavier than air, whose average molar mass is 28.8. According to the ideal gas law, NO2 is therefore more dense than air.
The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order of one and a quarter, as in ozone (O3).
The ground electronic state of nitrogen dioxide is a doublet state, since there is one unpaired bonding electron delocalised over both bonds, hence the bond order of one and a quarter.
Occurrence
NO2 exists in equilibrium with dinitrogen tetroxide (N2O4):
The equilibrium is characterized by ΔH = −57.23 kJ/mol, which is exothermic. Resulting from an endergonic reaction at higher temperatures, the paramagnetic monomer is favored. Colourless diamagnetic N2O4 can be obtained as a solid melting at m.p. −11.2 °C.[2]
Preparation and reactions
Nitrogen dioxide typically arises via the oxidation of nitric oxide by oxygen in air:[2]
- 2 NO + O2 → 2 NO2
In the laboratory, NO2 can be prepared in a two step procedure by thermal decomposition of dinitrogen pentoxide, which is obtained by dehydration of nitric acid:
- 2 HNO3 → N2O5 + H2O
- 2 N2O5 → 4 NO2 + O2
The thermal decomposition of some metal nitrates also affords NO2:
- 2 Pb(NO3)2 → 2 PbO + 4 NO2 + O2
Alternatively, reduction of concentrated nitric acid by metal (such as copper).
- 4 HNO3 + Cu → Cu(NO3)2 + 2 NO2 +2 H2O
Main reactions
The chemistry of nitrogen dioxide has been investigated extensively. At 150 °C, NO2 decomposes with release of oxygen via an endothermic process (ΔH = 114 kJ/mol):
- 2 NO2 → 2 NO + O2
As suggested by the weakness of the N–O bond, NO2 is a good oxidizer. Consequently, it will combust, sometimes explosively, with many compounds, such as hydrocarbons.
It hydrolyzes with disproportionation to give nitric acid:
- 3 NO2 + H2O → NO + 2 HNO3
This reaction is one step in the Ostwald process for the industrial production of nitric acid from ammonia.[3] Nitric acid decomposes slowly to nitrogen dioxide, which confers the characteristic yellow color of most samples of this acid:
- 4 HNO3 → 4 NO2 + 2 H2O + O2
NO2 is used to generate anhydrous metal nitrates from the oxides:[2]
- MO + 3 NO2 → 2 M(NO3)2 + NO
Alkyl and metal iodides give the corresponding nitrites:
- 2 CH3I + 2 NO2 → 2 CH3NO2 + I2
- TiI4 + 4 NO2 → Ti(NO2)4 + 2 I2
The pure gas is produced by adding concentrated nitric acid over tin. Stannic acid is produced as byproduct.
4HNO3 + Sn → H2O + H2SnO3 + 4 NO2
Safety and pollution considerations
Nitrogen dioxide is toxic by inhalation. However, as the compound is acrid and easily detectable by smell at low concentrations, inhalation exposure can generally be avoided. One potential source of exposure is fuming nitric acid, which spontaneously produces NO2 above 0 °C. Symptoms of poisoning (lung edema) tend to appear several hours after inhalation of a low but potentially fatal dose. Also, low concentrations (4 ppm) will anesthetize the nose, thus creating a potential for overexposure.
The is some evidence that long-term exposure to NO2 at concentrations above 40–100 µg/m3 may decrease lung function and increase the risk of respiratory symptoms.[4]
Nitrogen dioxide is formed in most combustion processes using air as the oxidant. At elevated temperatures nitrogen combines with oxygen to form nitric oxide:
- O2 + N2 → 2 NO
Nitric oxide can be oxidized in air to form nitrogen dioxide. At normal atmospheric concentrations this is a very slow process.
- 2 NO + O2 → 2 NO2
The most important sources of NO2 are internal combustion engines,[5] thermal power stations and, to a lesser extent, pulp mills. Butane gas heaters and stoves are also sources. The excess air required for complete combustion of fuels in these processes introduces nitrogen into the combustion reactions at high temperatures and produces nitrogen oxides (NOx). Limiting NOx production demands the precise control of the amount of air used in combustion. In households, kerosene heaters and gas heaters are sources of nitrogen dioxide.
Nitrogen dioxide is also produced by atmospheric nuclear tests, and is responsible for the reddish colour of mushroom clouds.[citation needed]
Nitrogen dioxide is a large scale pollutant, with rural background ground level concentrations in some areas around 30 µg/m3, not far below unhealthy levels. Nitrogen dioxide plays a role in atmospheric chemistry, including the formation of tropospheric ozone. A 2005 study by researchers at the University of California, San Diego, suggests a link between NO2 levels and Sudden Infant Death Syndrome.[6]
Nitrogen dioxide is also produced naturally during electrical storms. The term for this process is "atmospheric fixation of nitrogen". The rain produced during such storms is especially good for the garden as it contains trace amounts of fertilizer. (Henry Cavendish 1784, Birkland -Eyde Process 1903, et-al)
See also
 Nitrogen dioxide (NO2) gas converts to the colorless gas dinitrogen tetroxide (N2O4) at low temperatures, and converts back to NO2 at higher temperatures. The bottles in this photograph contain equal amounts of gas at different temperatures.
Nitrogen dioxide (NO2) gas converts to the colorless gas dinitrogen tetroxide (N2O4) at low temperatures, and converts back to NO2 at higher temperatures. The bottles in this photograph contain equal amounts of gas at different temperatures.
- Nitrite
- Dinitrogen tetroxide
- Nitryl
- Nitrous oxide (N2O), "laughing gas", a linear molecule, isoelectronic with CO2 but with a nonsymmetric arrangement of atoms (NNO)
- Nitric oxide (NO), a problematic pollutant that is short lived because it converts to NO2 in the presence of free oxygen.
External links
- International Chemical Safety Card 0930
- National Pollutant Inventory - Oxides of nitrogen fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- WHO-Europe reports: Health Aspects of Air Pollution (2003) (PDF) and "Answer to follow-up questions from CAFE (2004) (PDF)
- Nitrogen Dioxide Air Pollution
- Nitrogen dioxide pollution in the world (image)
- A review of the acute and long term impacts of exposure to nitrogen dioxide in the United Kingdom IOM Research Report TM/04/03
References
- ^ a b "nitrogen dioxide (CHEBI:33101)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. 13 January 2008. Main. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=33101. Retrieved 4 October 2011.
- ^ a b c Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Michael Thiemann, Erich Scheibler, Karl Wilhelm Wiegand “Nitric Acid, Nitrous Acid, and Nitrogen Oxides” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim.
- ^ Health Aspects of Air Pollution with Particulate Matter,Ozone and Nitrogen Dioxide. World Health Organization. 13–15 January 2003. p. 48. http://www.euro.who.int/document/e79097.pdf. Retrieved 2011-11-19.
- ^ Son, Busoon; Wonho Yang, Patrick Breysse, Taewoong Chung and Youngshin Lee (March 2004). "Estimation of occupational and nonoccupational nitrogen dioxide exposure for Korean taxi drivers using a microenvironmental model". Environmental Research 94 (3): 291–296. doi:10.1016/j.envres.2003.08.004. PMID 15016597. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WDS-49WMV2W-1&_user=432163&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000020718&_version=1&_urlVersion=0&_userid=432163&md5=1568528cb723b88921f97d88ebddd336. Retrieved 2008-02-25.
- ^ "Sids Linked to Nitrogen Dioxide Pollution". http://www.medicineonline.com/news/12/1110/Sids-Linked-to-Nitrogen-Dioxide-Pollution.html. Retrieved 2008-02-25.
Categories:- Inorganic nitrogen compounds
- Oxides
- Bleaches
- Hazardous air pollutants
- Smog
- Free radicals
- O=[N]=O
Wikimedia Foundation. 2010.