- Dinitrogen tetroxide
-
Dinitrogen tetroxide  Dinitrogen tetraoxideOther namesDinitrogen(II) oxide(-I)
Dinitrogen tetraoxideOther namesDinitrogen(II) oxide(-I)Identifiers CAS number 10544-72-6 
PubChem 25352 ChemSpider 23681 
EC number 234-126-4 UN number 1067 ChEBI CHEBI:29803 
RTECS number QW9800000 Jmol-3D images Image 1 - [O-][N+](=O)[N+]([O-])=O
Properties Molecular formula N2O4 Molar mass 92.011 g/mol Appearance colourless gas Density 1.443 g/cm3 (liquid, 21 °C) Melting point −11.2 °C (261.9 K)
Boiling point 21.1 °C (294.3 K)
Solubility in water reacts Vapor pressure 96 kPa (20 °C)[1] Refractive index (nD) 1.00112 Structure Molecular shape planar, D2h Dipole moment zero Thermochemistry Std enthalpy of
formation ΔfHo298-19.5 kJ/mol Standard molar
entropy So298150.38 J K−1 mol−1 Hazards MSDS External MSDS EU Index 007-002-00-0 EU classification Very toxic (T+)
Corrosive (C)R-phrases R26, R34 S-phrases (S1/2), S9, S26, S28, S36/37/39, S45 NFPA 704 Flash point Non-flammable Related compounds Related nitrogen oxides Nitrous oxide
Nitric oxide
Dinitrogen trioxide
Nitrogen dioxide
Dinitrogen pentoxide tetroxide (verify) (what is:
tetroxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dinitrogen tetroxide (nitrogen tetroxide or nitrogen peroxide) is the chemical compound N2O4. It forms an equilibrium mixture with nitrogen dioxide; some call this mixture dinitrogen tetroxide, while some call it nitrogen dioxide. Dinitrogen tetroxide is a powerful oxidizer, highly toxic and corrosive. N2O4 is hypergolic with various forms of hydrazine, i.e., they burn on contact without a separate ignition source, making them popular bipropellant rocket fuels. It is a useful reagent in chemical synthesis.
Contents
Structure and properties
Dinitrogen tetroxide forms an equilibrium mixture with nitrogen dioxide. [2] The molecule is planar with an N-N bond distance of 1.78 Å and N-O distances of 1.19 Å. Unlike NO2, N2O4 is diamagnetic.[3] It is also colorless but can appear as a brownish yellow liquid due to the presence of NO2 according to the following equilibrium:
- N2O4 ⇌ 2 NO2
Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide.
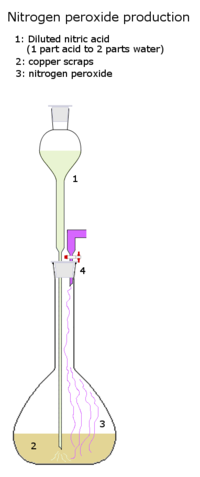
Production
Nitrogen dioxide is made by the catalytic oxidation of ammonia: steam is used as a diluent to reduce the combustion temperature. Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, and the remainder of the water is removed as nitric acid. The gas is essentially pure nitrogen tetroxide, which is condensed in a brine-cooled liquefier.[citation needed]
Use as a rocket propellant
Dinitrogen tetroxide is one of the most important rocket propellants ever developed, much like the German-developed hydrogen peroxide–based T-Stoff oxidizer used in their World War II rocket-propelled combat aircraft designs such as the Messerschmitt Me 163 Komet, and by the late 1950s it became the storable oxidizer of choice for rockets in both the USA and USSR. It is a hypergolic propellant often used in combination with a hydrazine-based rocket fuel. One of the earliest uses of this combination was on the Titan rockets used originally as ICBMs and then as launch vehicles for many spacecraft. Used on the U.S. Gemini, Apollo spacecraft and the Space Shuttle, it continues to be used on most geo-stationary satellites, and many deep-space probes. It now seems likely that NASA will continue to use this oxidizer in the next-generation 'crew-vehicles' which will replace the shuttle. It is also the primary oxidizer for Russia's Proton rocket and China's Long March rockets.
When used as a propellant, dinitrogen tetroxide is usually referred to simply as 'Nitrogen Tetroxide' and the abbreviation 'NTO' is extensively used. Additionally, NTO is often used with the addition of a small percentage of nitric oxide, which inhibits stress-corrosion cracking of titanium alloys, and in this form, propellant-grade NTO is referred to as "Mixed Oxides of Nitrogen" or "MON". Most spacecraft now use MON instead of NTO; for example, the Space Shuttle reaction control system uses MON3 (NTO containing 3wt%NO). [1]
On 24 July 1975, NTO poisoning nearly killed the three U.S. astronauts on board the Apollo-Soyuz Test Project during its final descent. This was due to a switch left in the wrong position, which allowed NTO fumes to vent out of the Apollo spacecraft then back in through the cabin air intake from the outside air after the external vents were opened. One crewmember lost consciousness during descent. Upon landing, the crew was hospitalized 14 days for chemical-induced pneumonia and edema.[4]
Power generation using N2O4
The tendency of N2O4 to reversibly break into NO2 has led to research into its use in advanced power generation systems as a so-called dissociating gas. "Cool" nitrogen tetroxide is compressed and heated, causing it to dissociate into nitrogen dioxide at half the molecular weight. This hot nitrogen dioxide is expanded through a turbine, cooling it and lowering the pressure, and then cooled further in a heat sink, causing it to recombine into nitrogen tetroxide at the original molecular weight. It is then much easier to compress to start the entire cycle again. Such dissociative gas Brayton cycles have the potential to considerably increase efficiencies of power conversion equipment.
Chemical reactions
Intermediate in the manufacture of nitric acid
Nitric acid is manufactured on a large scale via N2O4. This species reacts with water to give both nitrous acid and nitric acid:
- N2O4 + H2O → HNO2 + HNO3
The coproduct HNO2 upon heating disproportionates to NO and more nitric acid. When exposed to oxygen, NO is converted back into nitrogen dioxide:
- 2 NO + O2 → 2 NO2
The resulting NO2 (and N2O4, obviously) can be returned to the cycle to give the mixture of nitrous and nitric acids again.
Synthesis of metal nitrates
N2O4 behaves as the salt [NO+][NO3−], the former being a strong oxidant:
- 2 N2O4 + M → 2 NO + M(NO3)2
If metal nitrates are prepared from NTO in completely anhydrous conditions, a range of covalent metal nitrates can be formed with many transition metals. This is because there is a thermodynamic preference for the nitrate ion to bond covalently with such metals rather than form an ionic structure. Such compounds must be prepared in anhydrous conditions, since the nitrate ion is a much weaker ligand than water, and if water is present the simple hydrated nitrate will form. The anhydrous nitrates concerned are themselves covalent, and many, e.g. anhydrous copper nitrate, are volatile at room temperature. Anhydrous titanium nitrate sublimes in vacuum at only 40 degrees C. Many of the anhydrous transition metal nitrates have striking colours. This branch of chemistry was developed by Clifford Addisson and Noramn Logan at Nottingham University in the UK during the 1960s and 1970s when highly efficient desiccants and dry boxes started to become available.
See also: Nitrosonium tetrafluoroborateSee also
External links
- International Chemical Safety Card 0930
- National Pollutant Inventory - Oxides of nitrogen fact sheet
- NIOSH Pocket Guide to Chemical Hazards: Nitrogen tetroxide
- Air Liquide Gas Encyclopedia: NO2 / N2O4
References
- ^ International Chemical Safety Card
- ^ Henry A. Bent Dimers of Nitrogen Dioxide. II. Structure and Bonding Inorg. Chem., 1963, 2 (4), pp 747–752
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Sotos, John G., MD. "Astronaut and Cosmonaut Medical Histories", May 12, 2008, accessed April 1, 2011.
Categories:- Inorganic nitrogen compounds
- Oxides
- Rocket oxidizers
- Nitrates
- Oxidizing agents
Wikimedia Foundation. 2010.