- Nitrosonium tetrafluoroborate
-
Nitrosonium tetrafluoroborate 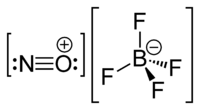 nitrosonium tetrafluoroborateOther namesnitrosyl tetrafluoroborate
nitrosonium tetrafluoroborateOther namesnitrosyl tetrafluoroborateIdentifiers CAS number 14635-75-7 PubChem 151929 Properties Molecular formula BF4NO Molar mass 116.81 g mol−1 Appearance colourless crystalline solid Density 2.185 g cm−3 Melting point 250 °C (sublimes)
Solubility in water decomposes  tetrafluoroborate (verify) (what is:
tetrafluoroborate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid finds use in organic synthesis as a nitrosating agent.[1]
NOBF4 is the nitrosonium salt of fluoroboric acid, and is composed of a nitrosonium cation, [NO]+, and a tetrafluoroborate anion, [BF4]−.
Reactions
Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type [MII(CH3CN)x][BF4]2 (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas:[2] With ferrocene the ferrocenium tetrafluoroborate is formed.[3]
- M + NOBF4 + xCH3CN → [M(CH3CN)x](BF4)2 + NO
References
- ^ "A15806 Nitrosonium tetrafluoroborate, 98%". Alfa Aesar website. http://www.alfa.com/en/GP100W.pgm?DSSTK=A15806. Retrieved 2010-09-04.
- ^ Robert A. Heintz, Jennifer A. Smith, Paul S. Szalay, Amy Weisgerber, And Kim R. Dunbar. "11. Homoleptic Transition Metal Acetonitrile Cations with Tetrafluoroborate or Trifluoromethanesulfonate Anions". Inorg. Synth. 33: 75–83. doi:10.1002/0471224502.ch2.
- ^ Roger M. Nielson, George E. McManis, Lance K. Safford, Michael J. Weaver (1989). "Solvent and electrolyte effects on the kinetics of ferrocenium-ferrocene self-exchange. A reevaluation". J. Phys. Chem. 93 (5): 2152. doi:10.1021/j100342a086.
Categories:- Inorganic compound stubs
- Tetrafluoroborates
- Nitrosyl compounds
Wikimedia Foundation. 2010.
