- Coronene
-
Coronene 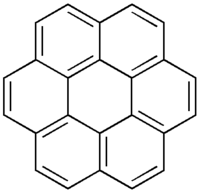
 Other names[6]circulene
Other names[6]circulene
X1001757-9, superbenzeneIdentifiers CAS number 191-07-1 
PubChem 9115 ChemSpider 8761 
KEGG C19375 
ChEBI CHEBI:29863 
Jmol-3D images Image 1 - c1cc2ccc3ccc4ccc5ccc6ccc1c7c2c3c4c5c67
Properties Molecular formula C24H12 Molar mass 300.35 g mol−1 Melting point 438 °C, 711 K, 820 °F
Boiling point 525 °C, 798 K, 977 °F
Solubility in water 0.14 μg/L  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Coronene (also known as superbenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising six peri-fused benzene rings.[1] Its chemical formula is C24H12. It is a yellow material that dissolves in such solvents as benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. Its emission spectrum is not symmetrical with its excitation spectrum and varies in the number of bands and their relative intensities depending on the solvent. It has been used as a solvent probe, similar to pyrene.
It occurs naturally as the mineral carpathite, which is characterized by flakes of pure coronene embedded in sedimentary rock. This mineral may result from ancient hydrothermal vent activity.[2][3]
Coronene is produced in the petroleum refining process of hydrocracking, where it can dimerize to a fifteen ring PAH, trivially named "dicoronylene" (formally named benzo[10,11]phenanthro[2',3',4',5',6':4,5,6,7]chryseno[1,2,3-bc]coronene or benzo[1,2,3-bc:4,5,6-b'c']dicoronene). Coronene has been seen on the surface of Titan[citation needed] (Saturn's moon). It can cause DNA damage in mammals.[citation needed]
The compound is of theoretical interest to organic chemists because of its aromaticity. It can be described by 20 resonance structures or by a set of three mobile Clar sextets. In the Clar sextet case, the most stable structure for coronene has only the three isolated outer sextets as fully aromatic although superaromaticity would still be possible when these sextets are able to migrate into the next ring.
According to in silico calculations on the following hypothetical isodesmic reactions, coronene has a somewhat higher resonance energy per pi electron (REPE) than benzene [4]:
- coronene + 24 * ethylene → 18 * butadiene REPE 4.2 kcal/mol (17.6 kJ/mol)
- benzene + 3 * ethylene → 3 * butadiene REPE 3.6 kcal/mol (15 kJ/mol)
On the other hand the perimeter C–C bond length alternations are larger than in benzene.
Hexa-benzopericoronenes
Hexa-benzopericoronenes are members of the coronene family and investigated in supramolecular electronics. They are known to self-assemble into a columnar phase. One derivative in particular forms carbon nanotubes with interesting electrical properties.[5] The columnar phase in this compound further organises itself into sheets, which ultimately roll up like a carpet to form multi-walled nanotubes with an outer diameter of 20 nanometers and a wall thickness of 3 nanometers. In this geometry, the stacks of coronene disks are aligned with the length of the tube. The nanotubes have sufficient length to fit between two platinum nanogap electrodes produced by scanning probe nanofabrication and are 180 nanometer apart. The nanotubes as such are insulating, but, after one-electron oxidation with nitrosonium tetrafluoroborate (NOBF4), they conduct electricity.
Organic synthesis of a hexa-benzopericoronene starts with an Aldol condensation reaction of an acetone derivative with a benzil derivative to substituted cyclopentadienone. This compound is reacted with an alkyne in a Diels-Alder reaction and subsequent expulsion of carbon monoxide to the hexaphenylbenzene, which is oxidized by Iron(III) chloride in nitromethane.
See also
- A related compound lacking the central core: cyclooctadecanonaene
- Coronene's dimer: Dicoronylene
References
- ^ Fetzer, J. C. (2000). The Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons. New York: Wiley.
- ^ www.luminousminerals.com
- ^ www.mindat.org
- ^ Super-Delocalized Valence Isomer of Coronene Arkadiusz Ciesielski, Micha K. Cyraski, Tadeusz M. Krygowski, Patrick W. Fowler, and Mark Lillington J. Org. Chem.; 2006; 71(18) pp 6840 - 6845; (Article) doi:10.1021/jo060898w
- ^ Jonathan P. Hill, Wusong Jin, Atsuko Kosaka, Takanori Fukushima, Hideki Ichihara, Takeshi Shimomura, Kohzo Ito, Tomihiro Hashizume, Noriyuki Ishii, Takuzo Aida (2004). "Self–Assembled Hexa-peri-hexabenzocoronene Graphitic Nanotube". Science 304 (5676): 1481–1483. doi:10.1126/science.1097789. PMID 15178796. http://www.sciencemag.org/cgi/data/304/5676/1481/DC1/1.
2 rings 3 rings 4 rings 5 rings 6+ rings Anthanthrene · Benzo[ghi]perylene · Corannulene · Coronene · Dicoronylene · Diindenoperylene · Helicene · Heptacene · Hexacene · Kekulene · Ovalene · ZethreneCategories:- Polycyclic aromatic hydrocarbons
Wikimedia Foundation. 2010.

