- Tetracene
-
For the initiating explosive, see tetrazene.
Tetracene 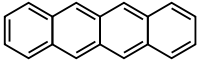
 TetraceneOther namesNaphthacene
TetraceneOther namesNaphthacene
Benz[b]anthracene
2,3-BenzanthraceneIdentifiers CAS number 92-24-0 
PubChem 7080 ChemSpider 6813 
ChEBI CHEBI:32600 
Jmol-3D images Image 1 - c34cc2cc1ccccc1cc2cc3cccc4
Properties Molecular formula C18H12 Molar mass 228.29 g/mol Appearance Yellow to orange solid Melting point 357 °C, 630 K, 675 °F
Solubility in water Insoluble Hazards EU Index Not listed  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tetracene, also called naphthacene, is a polycyclic aromatic hydrocarbon. It has the appearance of a pale orange powder. Tetracene is the four-ringed member of the series of acenes, the previous one being anthracene (tricene) and the next one being pentacene.
Tetracene is a molecular organic semiconductor, used in organic field-effect transistors (OFETs) and organic light-emitting diodes (OLEDs). In May 2007, researchers from two Japanese universities, Tohoku University in Sendai and Osaka University, have reported an ambipolar light-emitting transistor made of a single tetracene crystal.[1] Ambipolar means that the electric charge is transported by both, positively charged holes and negatively charged electrons. Tetracene can be also used as a gain medium in dye lasers as a sensitiser in chemoluminescence.
Jan Hendrik Schön during his time at Bell Labs (1997–2002) claimed to have developed an electrically pumped laser based on tetracene. However, his results could not be reproduced, and it is considered to be scientific fraud.[2]
References
- ^ T. Takahashi, T. Takenobu, J. Takeya, Y. Iwasa (2007). "Ambipolar Light-Emitting Transistors of a Tetracene Single Crystal". Advanced Functional Materials 17 (10): 1623–1628. doi:10.1002/adfm.200700046. http://www3.interscience.wiley.com/cgi-bin/abstract/114266185/ABSTRACT.
- ^ Agin, Dan (2007). Junk Science: An Overdue Indictment of Government, Industry, and Faith Groups That Twist Science for Their Own Gain. Macmillan. ISBN 9780312374808. http://books.google.com/?id=VxcjOL1j8iAC.
2 rings 3 rings 4 rings Benz[a]anthracene · Benzo[a]fluorene · Benzo[c]phenanthrene · Chrysene · Fluoranthene · Pyrene · Tetracene · Triphenylene5 rings 6+ rings Anthanthrene · Benzo[ghi]perylene · Corannulene · Coronene · Dicoronylene · Diindenoperylene · Helicene · Heptacene · Hexacene · Kekulene · Ovalene · ZethreneCategories:- Polycyclic aromatic hydrocarbons
- Organic semiconductors
- Laser gain media
Wikimedia Foundation. 2010.
