- Adiponitrile
-
Adiponitrile 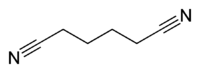 HexanedinitrileOther names1,4-Dicyanobutane, Adipic acid dinitrile, Adipic acid nitrile, Hexanedioic acid dinitrile, Adipyldinitrile, Nitrile adipico, Tetramethylene cyanide, Tetramethylene dicyanide, UN 2205
HexanedinitrileOther names1,4-Dicyanobutane, Adipic acid dinitrile, Adipic acid nitrile, Hexanedioic acid dinitrile, Adipyldinitrile, Nitrile adipico, Tetramethylene cyanide, Tetramethylene dicyanide, UN 2205Identifiers CAS number 111-69-3 
ChemSpider 13876621 
UNII VK98I9YW5M 
Jmol-3D images Image 1 - N#CCCCCC#N
Properties Molecular formula C6H8N2 Molar mass 108.14 g mol−1 Appearance Colourless oil Density 0.97 g/cm3 Melting point 1 °C
Boiling point 295 °C
Solubility in water 50 g/L (20 °C) Vapor pressure 0.003 hPa (20 °C) Hazards R-phrases R23, R25, R36, R38 S-phrases S26, S37, S45 Main hazards Toxic, irritant NFPA 704 Flash point 93 °C (decomposition) Autoignition
temperature460 °C Explosive limits 1.7 - 4.9 %V  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Adiponitrile is the organic compound with the formula (CH2)4(CN)2. This dinitrile, a viscous, colourless liquid, is an important precursor to the polymer nylon 66. In 2005, about one billion kilograms were produced annually.[1]
Contents
Production
Early routes
Because of the industrial value of adiponitrile, many methods have been developed for its synthesis. Early industrial routes started from furfural and later by the chlorination of butadiene to give 1,4-dichloro-2-butene, which with sodium cyanide, converts to 3-hexenedinitrile, which in turn can be hydrogenated to adiponitrile:[1]
- ClCH2CH=CHCH2Cl + 2 NaCN → NCCH2CH=CHCH2CN + 2 NaCl
- NCCH2CH=CHCH2CN + H2 → NC(CH2)4CN
Adiponitrile has also been produced from adipic acid, by dehydration of the diamide, but this route is rarely employed.
Modern routes
The majority of adiponitrile is prepared by the nickel-catalysed hydrocyanation of butadiene, as discovered at duPont. The net reaction is:
- CH2=CHCH=CH2 + 2 HCN → NCCH2CH2CH2CH2CN
The process involves several stages, the first of which involves monohydrocyanation (addition of one molecule of HCN), affording isomers of pentenenitriles as well as 2- and 3-methylbutenenitriles. These unsaturated nitriles are subsequently isomerized to the 3-and 4-pentenenitriles. In the final stage, these pentenenitriles are subjected to a second hydrocyanation, in an anti-Markovnikov sense, to produce adiponitrile.[1]
Research has shown that the 3-pentenenitrile, formed in the first hydrocyanation, can undergo alkene metathesis to give dicyanobutenes, which are readily hydrogenated as described above.
The other major industrial route involves electrosynthesis, starting from acrylonitrile, which is dimerized:
- 2 CH2=CHCN + 2 e- + 2 H+ → NCCH2CH2CH2CH2CN
The electrolytic coupling of acrylonitrile was discovered at Monsanto Company.
Applications
Almost all adiponitrile is hydrogenated to 1,6-diaminohexane for the production of nylon:[2]
- NC(CH2)4CN + 4 H2 → H2N(CH2)6NH2
Like other nitriles, adiponitrile is susceptible to hydrolysis. The resulting adipic acid however is more cheaply prepared by other routes.
Safety
The LD50 for adiponitrile is 300 mg/kg for oral ingestion by rats.[1]
In 1990, ACGIH adopted a time weighted average Threshold Limit Value of 2ppm for work related skin exposure. [3]
The NIOSH recommended skin exposure limit for a work related time weighted average concentration is 4ppm (18mg/m3). [4]
References
- ^ a b c d M. T. Musser, "Adipic Acid" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a01 269
- ^ Robert A. Smiley "Hexamethylenediamine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a12 629
- ^ 2009 TLVs and BEIs, American Conference of Governmental Industrial Hygienists, Signature Publications, page 11 of 254.
- ^ NIOSH Pocket Guide NIOSH Publication 2005-149; September 2005
External links
Categories:- Nitriles
Wikimedia Foundation. 2010.

