- Galantamine
-
Galantamine 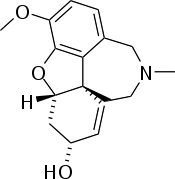
Systematic (IUPAC) name (4aS,6R,8aS)- 5,6,9,10,11,12- hexahydro- 3-methoxy- 11-methyl- 4aH- [1]benzofuro[3a,3,2-ef] [2] benzazepin- 6-ol Clinical data Trade names Razadyne AHFS/Drugs.com monograph MedlinePlus a699058 Pregnancy cat. B Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability 80 to 100% Protein binding 18% Metabolism Hepatic partially CYP450:CYP2D6/3A4 substrate Half-life 7 hours Excretion Renal (95%, of which 32% unchanged), fecal (5%) Identifiers CAS number 357-70-0 
ATC code N06DA04 PubChem CID 9651 DrugBank APRD00206 ChemSpider 9272 
UNII 0D3Q044KCA 
KEGG D04292 
ChEBI CHEBI:42944 
ChEMBL CHEMBL659 
Chemical data Formula C17H21NO3 Mol. mass 287.354 g/mol SMILES eMolecules & PubChem Physical data Melt. point 126.5 °C (260 °F)  (what is this?) (verify)
(what is this?) (verify)Galantamine (Nivalin, Razadyne, Razadyne ER, Reminyl, Lycoremine) is used for the treatment of mild to moderate Alzheimer’s disease and various other memory impairments, in particular those of vascular origin. It is an alkaloid that is obtained synthetically or from the bulbs and flowers of Galanthus Caucasicus (Caucasian snowdrop, Voronov’s snowdrop), Galanthus woronowii (Amaryllidaceae) and related genera like Narcissus (daffodil))[1], Leucojum (snowflake), and Lycoris including Lycoris radiata (Red Spider Lily).
Studies of usage in modern medicine began in the Soviet Union in the 1950s. The active ingredient was extracted, identified, and studied, in particular in relation to its acetylcholinesterase (AChE)-inhibiting properties. The bulk of the work was carried out by Soviet pharmacologists Mashkovsky and Kruglikova-Lvova, beginning in 1951.[2] The work of Mashkovsky and Kruglikova-Lvova was the first published work that demonstrated the AChE-inhibiting properties of galantamine.[3]
The first industrial process was developed in Bulgaria by prof. Paskov in 1959 (Nivalin, Sopharma) from a species traditionally used as a popular medicine in Eastern Europe, and, thus ,the idea for developing a medicine from these species seems to be based on the local use (i.e., an ethnobotany-driven drug discovery).[4][5]
Galantamine has been used for decades in Eastern Europe and the USSR for various indications such as treatment of myasthenia, myopathy, and sensory and motor dysfunction associated with disorders of the central nervous system. Its uses have included symptomatic treatment of Polio (Poliomyelitis), and it was later deployed by Janssen Pharmaceutica as an anti-Alzheimer's medication. In the US, it has been sold as a dietary supplement for memory and dream support.
Contents
Pharmacology
Galantamine in its pure form is a white powder. Galantamine is a competitive and reversible cholinesterase inhibitor. It reduces the action of AChE and therefore tends to increase the concentration of acetylcholine in the brain. It is hypothesized that this action might relieve some of the symptoms of Alzheimer's. It is also an allosteric ligand at nicotinic acetylcholine receptors.
The atomic resolution 3D structure of the complex of galantamine and its target, acetylcholinesterase, was determined by X-ray crystallography in 1999 (PDB code: 1DX6; see complex).[6] There is no evidence that galantamine alters the course of the underlying dementing process.[7] Galantamine has also shown activity in modulating the nicotinic cholinergic receptors on cholinergic neurons to increase acetylcholine release.[8]
Pharmacokinetics
Absorption of galantamine is rapid and complete and shows linear pharmacokinetics. It is well absorbed with absolute oral bioavailability between 80 and 100%. It has a half-life of seven hours. Peak effect of inhibiting acetylcholinesterase was achieved about one hour after a single oral dose of 8 mg in some healthy volunteers.
Plasma protein binding of galantamine is about 18%, which is relatively low.
Metabolism
Approximately 75% of a dose of galantamine is metabolised in the liver. In vitro studies have shown that Hepatic CYP2D6 and CYP3A4 are involved in galantamine metabolism.
For Razadyne ER (the once-a-day formulation), CYP2D6 poor metabolizers had drug exposures that were approximately 50% higher than for extensive metabolizers. About 7% of the population has this genetic mutation; however, because the drug is individually titrated to tolerability, no specific dosage adjustment is necessary for this population.
Clinical use
Indications
Galantamine is indicated for the treatment of mild to moderate vascular dementia and Alzheimer's.[9][10]
Available forms
The product is supplied in twice-a-day tablets, in once-a-day extended-release capsules, and in oral solution. The tablets come in 4 mg, 8 mg, and 12 mg forms. The capsules come in 8 mg, 16 mg, and 24 mg forms.
Adverse events
In clinical trials, galantamine's side effect profile was very similar to that of other cholinesterase inhibitors, with gastrointestinal symptoms being the most notable and most commonly observed. In practice, some other cholinesterase inhibitors might be better tolerated; however, a careful and gradual titration over more than three months may lead to equivalent long-term tolerability.[11]
Other uses
Supplement for lucid dream and out-of-body experience
Some people who practice lucid dream (LD) or out-of-body experience (OBE) use galantamine to increase their odds to achieve LD or OBE.[12][13][14] By taking small amount of galantamine (around 4 to 8 mg) after five to six hours of deep sleep and practice an induction technique such as meditation, MILD or WILD [1] many people report more success with galantamine.[15]
There are also reports claiming that taking galantamine without proper induction technique will not lead to LD or OBE but will result in only a vivid dream instead. It should also be noted that, due to a long half-life, galantamine will stay in the body for a period of up to and over 48 hours. As such, it is advisable to space out the use of galantamine over a period of three days so that the body does not build a resistance to the drug, ruining its effectiveness.[12]
Galantamine used with choline bitartrate or Alpha-GPC can dramatically increase one's odds of becoming lucid and increase memory consolidation during dreaming.[citation needed] Some people report mixing galantamine with other nootropic can enhance the degree of lucidity, but this is still controversial, since some mixtures may work for some people, but lead to failure for others.
Sleep aid
Galantamine has been used as a sleep aid, Galantamine has been anecdotally described both to help people fall asleep and increasing the quality of sleep once someone falls asleep. Extensive research and sleep studies have not been conducted, but initial microbiological research suggests that Galantamine does have properties that can aid patients suffering from insomnia.
Nootropic
Along with other cholinergics or acetylcholinesterase inhibitors such as Huperzine A, galantamine also has been used as nootropic or "brain enhancer" to enhance memory in brain-damaged adults.[16]
Caution
The U.S. Food and Drug Administration (FDA) and international health authorities have published an alert based on data from two studies during the treatment by galantamine of mild cognitive impairment (MCI); higher mortality rates were seen in drug-treated patients.[17][18] On April 27, 2006, FDA approved labeling changes concerning all form of galantamine preparations (liquid, regular tablets,and extended release tablets) warning of the risk of bradycardia (and sometimes atrioventricular block, especially in predisposed persons). At the same time, the risk of syncope seems to be increased relative to placebo.[19] These side effects have not been reported in any other studies except[clarification needed] in mild cognitive impairment.
Total synthesis
Galantamine is produced from natural resources and a patented total synthesis process. Many other synthetic methods exist but have not been implemented on an industrial scale.
References
- ^ NNFCC Project Factsheet: Sustainable Production of the Natural Product Galanthamine (Defra), NF0612
- ^ Snowdrops: the heralds of spring and a modern drug for Alzheimer’s disease
- ^ Mashkovsky MD, Kruglikova-Lvova RP. On the pharmacology of the new alkaloid galantamine. Farmakologia Toxicologia (Moscow) 1951;14:27-30 (in Russian).
- ^ Heinrich, M.; Teoh, H.L. (2004). "Galanthamine from snowdrop – the development of a modern drug against Alzheimer's disease from local Caucasian knowledge". Journal of Ethnopharmacology 92 (2–3): 147–162. doi:10.1016/j.jep.2004.02.012. PMID 15137996.
- ^ Scott, LJ; Goa, KL (2000). "Galantamine: a review of its use in Alzheimer's disease". Drugs 60 (5): 1095–122. PMID 11129124.
- ^ Greenblatt, HM; Kryger, G; Lewis, T; Silman, I; Sussman, JL (1999). "Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3Å resolution". FEBS Lett 463 (3): 321–26. doi:10.1016/S0014-5793(99)01637-3. PMID 10606746.
- ^ Ortho-McNeil Neurologics, "Razadyne ER US Product Insert", May 2006
- ^ Woodruff-Pak, DS; Vogel Rw, 3rd; Wenk, GL (2001). "Galantamine: Effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning". Proceedings of the National Academy of Sciences of the United States of America 98 (4): 2089–94. doi:10.1073/pnas.031584398. PMC 29386. PMID 11172080. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=29386.
- ^ Galantamine Benefits Both Alzheimer’s Disease and Vascular Dementia
- ^ Galantamine Improves Attention in Alzheimer's
- ^ Birks, J; Birks, Jacqueline (2006). Birks, Jacqueline. ed. "Cholinesterase inhibitors for Alzheimer's disease". Cochrane database of systematic reviews (Online) (1): CD005593. doi:10.1002/14651858.CD005593. PMID 16437532.
- ^ a b Thomas Yuschak (2006). Advanced Lucid Dreaming (1st ed.). Lulu Enterprises. ISBN 978-1-4303-0542-2.
- ^ Thomas Yuschak (2007). Pharmacological Induction of Lucid dreams. http://www.advancedld.com/f/Pharmacological_Induction_of_Lucid_Dreams.pdf.
- ^ "Substances that enhance recall and lucidity during dreaming". Stephen LaBerge - US Patent. http://www.freepatentsonline.com/20040266659.html. Retrieved 2007-10-29.
- ^ "Galantamine LDS Profile". Yuschak LDS Profiles. http://www.advancedld.com/galantamine.html. Retrieved 2007-10-29.
- ^ Galantamine Protects Neurons and Memory Following Brain Injury
- ^ "FDA ALERT: Galantamine hydrobromide (marketed as Razadyne, formerly Reminyl) - Healthcare Professional Sheet". Postmarket Drug Safety Information for Patients and Providers. Food and Drug Administration. May 2005. http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126138.pdf. Retrieved 2010-04-02.
- ^ "Safety Alerts for Human Medical Products > Reminyl (galantamine hydrobromide)". # MedWatch The FDA Safety Information and Adverse Event Reporting Program. Food and Drug Administration. March 2005. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm152595.htm. Retrieved 2010-08-04.
- ^ "Safety Labeling Changes Approved By FDA Center for Drug Evaluation and Research (CDER)". MedWatch, The FDA Safety Information and Adverse Event Reporting Program. Food and Drug Administration. April 2006. Archived from the original on 2007-10-09. http://web.archive.org/web/20071009145003/http://www.fda.gov/medwatch/safety/2006/apr06.htm. Retrieved 2009-07-30.
External links
- Memeron (galantamine compound website)
- Razadyne (manufacturer's website)
- Galantamine (patient information)
- Proteopedia 1dx6
- AChE inhibitors and substrates (Part II)
Psychoanaleptics: Antidementia agents (N06D) Anticholinesterases Cymserine • Donepezil • Galantamine • Huperzine A (Huperzia serrata) • Ladostigil • Rivastigmine • TacrineOthers Bifemelane • Bilobalide (Ginkgo biloba) • Cerlapirdine • Ensaculin • Latrepirdine • Lecozotan • Leteprinim • Memantine • SemagacestatNootropics (N06B) Acetylcholinesterases Ampakines CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • Sunifiram • UnifiramD1 Agonists Eugeroics GABAA α5 Inverse Agonists H3 Antagonists mACh Agonists Alvameline • Arecoline • Cevimeline • CI-1017 • Milameline • Sabcomeline • Talsaclidine • Tazomeline • XanomelinenACh Agonists Racetams Others Acetylcarnitine • Adafenoxate • Bifemelane • Bilobalide (Ginkgo Biloba) • Carbenoxolone • Cerlapirdine • Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine • Ensaculin • Fipexide • Idebenone • Indeloxazine • Latrepirdine • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • Pirisudanol • Pyritinol • S-17092 • Sulbutiamine • Taltirelin • Teniloxazine • Tricyanoaminopropene • VinpocetineCholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Acetylcholinesterase inhibitors
- Antidementia agents
- Nootropics
- Phenol ethers
- Alcohols
- Benzazepines
- Janssen Pharmaceutica
Wikimedia Foundation. 2010.
Look at other dictionaries:
Galantamine — Structure de la galantamine Général Nom IUPAC 4aS,6R,8aS) 5,6,9,10,11,12 hexahydro 3 methoxy 11 methy … Wikipédia en Français
galantamine — /ga lanˈtə mēn/ noun A tertiary amine compound, orig derived from flowers, esp daffodils and snowdrops, used as a drug in treating dementia ORIGIN: Galanthus, snowdrop genus, and ↑amine … Useful english dictionary
galantamine — noun An alkaloid, obtained synthetically or from various plants, used for the treatment of mild to moderate Alzheimers disease and various memory impairments … Wiktionary
galantamine — n. see acetylcholinesterase inhibitor … Medical dictionary
galantamine — n.; see acetylcholinesterase inhibitor … The new mediacal dictionary
galantamine — n.f. Molécule utilisée pour traiter la maladie d Alzheimer … Le dictionnaire des mots absents des autres dictionnaires
galantamine hydrobromide — ga·lan·ta·mine hy·dro·bro·mide (gə lanґtə mēn) the hydrobromide salt of an alkaloid obtained from the daffodil Narcissus pseudonarcissus; it is a reversible competitive inhibitor of acetylcholinesterase and is administered orally… … Medical dictionary
357-70-0 — Galantamine Galantamine Structure de la galantamine Général Nom IUPAC 4aS,6R,8aS) 5,6,9,10,11,12 hexah … Wikipédia en Français
C17H21NO3 — Galantamine Galantamine Structure de la galantamine Général Nom IUPAC 4aS,6R,8aS) 5,6,9,10,11,12 hexah … Wikipédia en Français
Nicotinic agonist — A nicotinic agonist is a drug which enhances the action at the nicotinic acetylcholine receptor (nAChR). Examples include: nicotine (by definition the nicotinic acetylcholine receptor is named for its affinity for nicotine) acetylcholine, the… … Wikipedia
