- Tabun (nerve agent)
-
Tabun 
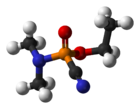 Ethyl N,N-DimethylphosphoramidocyanidateOther namesGA; Ethyl dimethylphosphoramidocyanidate; Dimethylaminoethoxy-cyanophosphine oxide; Dimethylamidoethoxyphosphoryl cyanide; Ethyl dimethylaminocyanophosphonate; Ethyl ester of dimethylphosphoroamidocyanidic acid; Ethyl phosphorodimethylamidocyanidate; Cyanodimethylaminoethoxyphosphine oxide; Dimethylaminoethodycyanophosphine oxide; EA1205
Ethyl N,N-DimethylphosphoramidocyanidateOther namesGA; Ethyl dimethylphosphoramidocyanidate; Dimethylaminoethoxy-cyanophosphine oxide; Dimethylamidoethoxyphosphoryl cyanide; Ethyl dimethylaminocyanophosphonate; Ethyl ester of dimethylphosphoroamidocyanidic acid; Ethyl phosphorodimethylamidocyanidate; Cyanodimethylaminoethoxyphosphine oxide; Dimethylaminoethodycyanophosphine oxide; EA1205Identifiers CAS number 77-81-6 
ChemSpider 6254 
ChEMBL CHEMBL446997 
Jmol-3D images Image 1 - N#CP(=O)(OCC)N(C)C
Properties Molecular formula C5H11N2O2P Molar mass 162.13 g mol−1 Appearance Colorless to brown liquid Density 1.0887 g/cm³ at 25 °C
1.102 g/cm³ at 20 °CMelting point -50 °C, 223 K, -58 °F
Boiling point 247.5 °C, 521 K, 478 °F
Solubility in water 9.8 g/100 g at 25 °C
7.2 g/100 g at 20 °CVapor pressure 0.07 mmHg (9 Pa) Hazards Main hazards Toxic. Fires involving this chemical may result in the formation of hydrogen cyanide NFPA 704 Flash point 78 °C  (nerve agent) (verify) (what is:
(nerve agent) (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tabun or GA is an extremely toxic chemical substance. It is a clear, colorless, and tasteless liquid with a faint fruity odor.[1] It is classified as a nerve agent because it fatally interferes with normal functioning of the mammalian nervous system. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687, and its production is strictly controlled and stockpiling outlawed by the Chemical Weapons Convention of 1993. Tabun is the first of the so-called G-series nerve agents along with GB (sarin), GD (soman) and GF (cyclosarin).
Although pure tabun is clear, less-pure tabun may be brown. It is a volatile chemical, although less so than either sarin or soman; because of this, tabun can be used to contaminate water.[1]
Tabun can be destroyed with bleaching powder, though the poisonous gas cyanogen chloride is produced.[2]
Contents
Synthesis
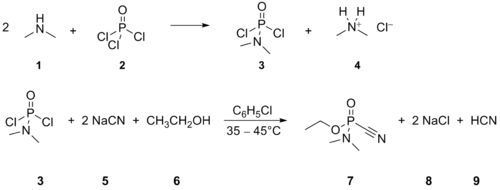
Tabun was manufactured on an industrial scale by Germany during World War II, based on a process developed by Dr. Gerhard Schrader. In the chemical agent factory in Dyhernfurth an der Oder, codenamed "Hochwerk", at least 12,000 metric tons of this agent were manufactured between 1942 and 1945. The manufacturing process consisted of two steps, first being reaction of gaseous dimethylamine (1) with an excess of phosphoryl chloride (2), yielding dimethylamidophosphoric dichloride (3, codenamed "Produkt 39" or "D 4") and dimethylammonium chloride (4). Dimethylamidophosphoric dichloride thus obtained was purified by vacuum distillation and thereafter transferred to the main Tabun production line. Here it was reacted with an excess of sodium cyanide (5), dispersed in dry chlorobenzene, yielding the intermediate dimethylamidophosphoric dicyanide (not depicted in the scheme) and sodium chloride (8); then, absolute ethanol (6) was added, reacting with the dimethylamidophosphoric dicyanide to yield tabun (7) and hydrogen cyanide (9). After the reaction, the mixture (consisting of about 75% chlorobenzene and 25% tabun, along insoluble salts and rests of hydrogen cyanide) was filtered so as to remove the insoluble salts and vacuum-distilled to remove hydrogen cyanide and excess chlorobenzene to finally yield the technical product, consisting either of 95% tabun with 5% chlorobenzene (Tabun A), or later in the war, 80% tabun with 20% chlorobenzene (Tabun B).[3]
Effects of overexposure
The symptoms of exposure include:[2][4][5] nervousness/restlessness, miosis (contraction of the pupil), rhinorrhea (runny nose), excessive salivation, dyspnea (difficulty in breathing due to bronchoconstriction/secretions), sweating, bradycardia (slow heartbeat), loss of consciousness, convulsions, flaccid paralysis, loss of bladder and bowel control, apnea (breathing stopped) and lung blisters. The exact symptoms of overexposure are similar to those created by all nerve agents. Tabun is toxic even in minute doses. The number and severity of symptoms which appear vary according to the amount of the agent absorbed and rate of entry of it into the body. Very small skin dosages sometimes cause local sweating and tremors accompanied with characteristically constricted pupils with few other effects. Tabun is about half as toxic as sarin by inhalation, but in very low concentrations it is more irritating to the eyes than sarin. Also, tabun breaks down slowly, which after repeated exposure can lead to build up in the body.[1]
The effects of tabun appear slowly when tabun is absorbed through the skin rather than inhaled. A victim may absorb a lethal dose quickly, although death may be delayed for one to two hours.[4] A person's clothing can release the toxic chemical for up to 30 minutes after exposure.[1] Inhaled lethal dosages kill in one to ten minutes, and liquid absorbed through the eyes kills almost as fast. However, people who experience mild to moderate exposure to tabun can recover completely, if treated almost as soon as exposure occurred.[1] The LCt50 for tabun is about 400 mg-min/m3[6]
Treatment for suspected tabun poisoning is often three injections of a nerve agent antidote, such as atropine.[5] Pralidoxime chloride (2-PAM Cl) also works as an antidote; however, it must be administered within minutes to a few hours following exposure to be effective.[7]
History
Tabun was the first nerve agent to be discovered by accident in January 1936[1][2][8][9][10] by the German researcher Gerhard Schrader.[10] Schrader was experimenting with a class of compounds called organophosphates, which kill insects by interrupting their nervous systems, in order to create a more effective insecticide for IG Farben, a German chemical and pharmaceutical industry conglomerate, at Elberfield. Instead of a new insecticide, he discovered tabun, a chemical enormously toxic to humans as well as insects.
During World War II, as part of the Grün 3 program, a plant for the manufacture of tabun was established at Dyhernfurth[10] (now Brzeg Dolny, Poland), in 1939. Run by Anorgana, GmbH, the plant finally began production of the substance in 1942.[10] The reason that the plant took so long to get started was the extreme precautions used by the plant.[10] Intermediate products of tabun were corrosive, and had to be contained in quartz or silver-lined vessels. Tabun itself was also highly toxic, and final reactions were conducted behind double glass walls.[10] Large scale manufacturing of the agent resulted in problems with tabun's degradation over time, and only around 12,500 tons of material were manufactured before the plant was seized by the Soviet Army. The plant initially produced shells and aerial bombs using a 95:5 mix of tabun and chlorobenzene, designated "Variant A", and in the latter half of the war switched to "Variant B," a 80:20 mix of tabun and chlorobenzene designed for easier dispersion. The Soviets dismantled the plant and shipped it to Russia.[citation needed]
The United States once had a tabun production program,[5] which ended many decades ago. Like the other Allied governments, the Soviets soon abandoned GA for GB and GD. Large quantities of the German-manufactured agent were dumped into the sea to neutralize the substance.
Since GA is much easier to produce than the other G-series weapons and the process is comparatively widely understood, countries that develop a nerve agent capability but lack advanced industrial facilities often start by producing GA.
In his 1970s-1980s tracts to the medias, U.S. conspiracy theorist Francis E. Dec often claimed the use of tabun (misspelled "tabin")[11] as a covert assassination tool in the U.S.
During the Iran–Iraq War, Iraq employed quantities of chemical weapons against Iranian ground forces. Although the most commonly used agents were mustard gas and sarin, tabun and cyclosarin were also used.[5][12]
See also
- Cyclosarin (GF)
- Deseret Chemical Depot - location of remaining US stockpile
- Nerve agent
- Sarin (GB)
- Soman (GD)
References
- ^ a b c d e f Facts About Tabun, National Terror Alert Response System
- ^ a b c "Nerve Agent: GA". Cbwinfo.com. http://www.cbwinfo.com/Chemical/Nerve/GA.shtml. Retrieved 2008-11-06.
- ^ Lohs, KH: Synthetische Gifte. 3., überarb. u. erg. Aufl., 1967, Deutscher Militärverlag, Berlin (East).
- ^ a b "Chemical Warfare Weapons Fact Sheets — Tabun — GA Nerve Agent". Usmilitary.about.com. http://usmilitary.about.com/library/milinfo/blchemical-3.htm. Retrieved 2008-11-06.
- ^ a b c d http://www.encyclopedia.com/doc/1G2-3403300733.html
- ^ "ATSDR — MMG: Nerve Agents: Tabun (GA); Sarin (GB); Soman (GD); and VX". Atsdr.cdc.gov. http://www.atsdr.cdc.gov/MHMI/mmg166.html. Retrieved 2008-11-06.
- ^ Emergency Response Safety and Health Database. TABUN (GA): Nerve Agent. National Institute for Occupatinal Safety and Health. Accessed April 30, 2009.
- ^ Chemical Warfare Weapons Fact Sheets, about.com
- ^ Chemical Weapons: Nerve Agents, University of Washington
- ^ a b c d e f "A Short History of the Development of Nerve Gases". Noblis.org. http://www.noblis.org/AShortHistoryOfTheDevelopmentOfNerveGases.htm. Retrieved 2008-11-06.
- ^ Branting, Peter (2008). The Dectionary, "Deadly Touch Tabin Needle, The" — A list explaining the recurring concepts found in Dec's flyers.
- ^ http://abcnews.go.com/US/story?id=90722&page=1
Further reading
- United States Senate, 103d Congress, 2d Session. (May 25, 1994). Material Safety Data Sheet—Lethal Nerve Agent Tabun (GA). Retrieved Nov. 6, 2004.
- United States Central Intelligence Agency (Jul. 15, 1996) Stability of Iraq's Chemical Weapon Stockpile
- Norris, John (1997). NBC: Nuclear, Biological and Chemical Warfare on the Modern Battlefield. Brassey's UK. p. 20. ISBN 1857531825.
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Anticholinesterases
- Nerve agents
- Nitriles
- Ethyl esters
Wikimedia Foundation. 2010.
Look at other dictionaries:
Nerve agent — This article is about the chemical. For the band, see The Nerve Agents. This article forms part of the series Chemical agents Lethal agents Blood agents Cyanogen chloride (CK) … Wikipedia
organophosphate nerve agent — noun any of a series of nerve agents containing organophosphate compounds first synthesized by German chemists in 1936; in World War II the Germans tested them in concentration camps but not on the battlefield; Iraq is alleged to have used them… … Useful english dictionary
Tabun — may refer to:* Tabun Cave, a cave near Tabun, Israel where remains of Neanderthal Man were found. * A tabun oven, a clay oven used to make tabun bread * Tabun (nerve agent), the first nerve agent chemical weapon to be discovered, just before the… … Wikipedia
nerve gas — noun a toxic gas that is inhaled or absorbed through the skin and has harmful effects on the nervous and respiratory system • Syn: ↑nerve agent • Hypernyms: ↑poison gas, ↑agent • Hyponyms: ↑VX gas, ↑organophosphate nerve agent … Useful english dictionary
tabun — noun the first known nerve agent, synthesized by German chemists in 1936; a highly toxic combustible liquid that is soluble in organic solvents and is used as a nerve gas in chemical warfare • Syn: ↑GA • Hypernyms: ↑organophosphate nerve agent … Useful english dictionary
tabun — noun An extremely toxic nerve agent; a clear, tasteless liquid, molecular formula CHNOP. Syn: GA … Wiktionary
nerve gas — any of several poison gases, derived chiefly from phosphoric acid, that weaken or paralyze the nervous system, esp. that part of the system controlling respiration. [1935 40] * * * Weapon of chemical warfare that affects the transmission of nerve … Universalium
Novichok agent — This article forms part of the series Chemical agents Lethal agents Blood agents Cyanogen chloride (CK) Hydrogen cyanide (AC) … Wikipedia
Chemical Agent Identification Set — A typical glass bottle from a type of CAIS known as a toxic gas set . This one contains sulfur mustard (HD). Chemical Agent Identification Sets (CAIS), known by several other names, were sets of glass vials or bottles that contained small amounts … Wikipedia
Blood agent — This article forms part of the series Chemical agents Lethal agents Blood agents Cyanogen chloride (CK) Hydrogen cyanide (AC) … Wikipedia