- Chloropicrin
-
Chloropicrin 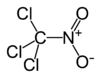
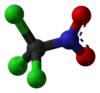 trichloro(nitro)methaneOther namesPS
trichloro(nitro)methaneOther namesPSIdentifiers CAS number 76-06-2 
ChemSpider 13861343 
UNII I4JTX7Z7U2 
KEGG C18445 
ChEBI CHEBI:39285 
Jmol-3D images Image 1 - ClC(Cl)(Cl)[N+]([O-])=O
Properties Molecular formula CCl3NO2 Molar mass 164.375 Appearance colorless liquid Density 1.692 g/ml[1] Melting point -69 °C
Boiling point 112 °C (dec)
Hazards Main hazards Extremely toxic and irritating to skin, eyes, and lungs.  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chloropicrin, also known as PS, is a chemical compound with the structural formula Cl3CNO2. This colourless highly toxic liquid was once used in chemical warfare and is currently used as a fumigant and nematocide.[2][3]
Contents
History
Chloropicrin was first discovered in 1848 by a Scottish chemist John Stenhouse. He prepared it by the reaction of a chlorinating agent with picric acid:
- HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl
Because of the precursor he used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar.
Arguably[weasel words], chloropicrin's most famous use was in World War I. In 1917, there were reports that the Germans were testing and using a new chemical in warfare.[4] That chemical was chloropicrin. While not as lethal as other chemical weapons, it caused vomiting and was a lachrymatory agent.[4] This combination of properties forced Allied soldiers to remove their masks to vomit, exposing them to toxic gases.[4] This caused a large number of casualties on the Italian front.[4]
Preparation
Chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite:[5]
- H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH
Properties
As listed in the Table, chloropicrin is a colorless liquid that is insoluble in water, with which it is stable. With a vapor pressure of 24 mm Hg, its volatility is between that of phosgene and mustard gas in persistency, although closer to phosgene because it is related to the compound.[6] Tests have shown that chloropicrin causes humans to shut their eyes involuntarily.[4] Chloropicrin can be absorbed systemically through inhalation, ingestion, and the skin. It is severely irritating to the lungs, eyes, and skin.[7] Because of these properties, chloropicrin can only be delivered in shell form as a chemical weapon.[6]
Application
Chloropicrin, today, is used as a fumigant to control pests found in the soil.[2] Although less common, it can be used as a poison for vertebrates, such as rabbits.[2] Chloropicrin is commonly used in combination with other fumigants, such as methyl bromide and sulfuryl fluoride, for increased potency and as a warning agent.[2]
Chloropicrin has been used in chemical warfare. It first appeared in 1917 when the Germans tested a new chemical weapon on the Italian front.[6] The new chemical weapon was devastating to the Allies at first, since they had never encountered it before.
Safety
Chloropicrin is a highly toxic chemical: NIOSH 1995 states that:
- Chloropicrin is a lacrimator and a severe irritant of the respiratory system in humans; it also causes severe skin irritation on contact. When splashed onto the eye chloropicrin has caused corneal oedema and liquification of the cornea.
- Exposure to concentrations of 15 ppm cannot be tolerated for more than 1 minute, and exposure to 4 ppm for a few seconds is temporarily disabling.
- Exposure to 0.3-0.37 ppm chloropicrin for 3 to 30 seconds causes tearing and eye pain. Exposure to 15 ppm for a few seconds can cause respiratory tract injury.
- Exposure to 119 ppm in air for 30 minutes is lethal; death is caused by pulmonary oedema.
Examples of industrial exposure in humans: 27 workers in a cellulose factory who were exposed to high levels of chloropicrin for 3 minutes developed pneumonitis after 3 to 12 hours of irritated coughing and difficulty on breathing; they subsequently developed pulmonary oedema, and one died.
EU classification of chloropicrin is: R22 Harmful if swallowed, R26 Very toxic by inhalation, R36/37/38 Irritating to eyes, skin and respiratory system, R43 May cause sensitisation by skin contact, R50/53 Very toxic to aquatic organisms, may cause long term adverse effects in the aquatic environment.
Because of chloropicrin's stability, protection requires highly effective absorbents, such as activated charcoal.[4] Chloropicrin, unlike its relative compound phosgene, is absorbed readily at any temperature, which may pose a threat in low or high temperature climates.[6]
The use of the substance has been restricted by the U.S. government, although such restriction is outdated now [8]
Portrayal in Media
In the 1987 Movie Dragnet, detectives Joe Friday (Dan Aykroyd) and Pep Streebeck (Tom Hanks) thwart an attempt by mad cultists to release a tanker truck of trichloronitromethane at a party attended by several prominent Los Angeles city officials.
The effects cited in explaining the substance (vomiting, suffocation, and death) are consistent with the actual chemical.
References
- ^ http://msds.chem.ox.ac.uk/CH/chloropicrin.html
- ^ a b c d http://www.workcover.nsw.gov.au/Documents/Publications/AlertsGuidesHazards/DangerousGoodsExplosivesFireworksPyrotechnics/chloropicrin_fact_sheet_1371.pdf
- ^ http://books.google.com/books?id=cTA6AAAAMAAJ&pg=PA144&dq=Chloropicrin+in+chemical+warfare#PPA144,M1
- ^ a b c d e f http://books.google.com/books?id=cTA6AAAAMAAJ&pg=PA144&dq=Chloropicrin+in+chemical+warfare&client=firefox-a#PPA144,M1
- ^ Sheldon B. Markofsky "Nitro Compounds, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- ^ a b c d http://books.google.com/books?id=cTA6AAAAMAAJ&pg=PA144&dq=Chloropicrin+in+chemical+warfare#PPA146,M1
- ^ Chloropicrin (PS): Lung Damaging Agent. National Institute for Occupational Safety and Health. Emergency Response Safety and Health Database, August 22, 2008. Retrieved December 23, 2008.
- ^ Environmental Protection Agency. "Restricted Use Products (RUP) Report: Six Month Summary List". http://www.epa.gov/opprd001/rup/rup6mols.htm. Retrieved 1 December 2009.
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Categories:- World War I chemical weapons
- Fumigants
- Pulmonary agents
- Lachrymatory agents
- Vomiting agents
- Nitromethanes
Wikimedia Foundation. 2010.
