- Cyanogen chloride
-
Cyanogen chloride 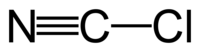
 Cyanic chlorideOther namesChloroformonitrile, Chlorine cyanide, Carbononitridic chloride, CK
Cyanic chlorideOther namesChloroformonitrile, Chlorine cyanide, Carbononitridic chloride, CKIdentifiers CAS number 506-77-4 
PubChem 10477 ChemSpider 10045 
EC number 208-052-8 UN number 1589 RTECS number GT2275000 Jmol-3D images Image 1 - ClC#N
Properties Molecular formula CNCl Molar mass 61.46 g/mol Density 1.186 g/cm3 (liquid) Melting point −6 °C
Boiling point 13 °C[1]
Solubility in water Soluble Vapor pressure 1987 kPa (21.1 °C) Hazards MSDS ICSC 1053 Main hazards Highly toxic[2] NFPA 704 Related compounds Related cyanogen halides Cyanogen fluoride
Cyanogen bromide
Cyanogen iodideRelated compounds Cyanogen  chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyanogen chloride is an inorganic compound with the formula NCCl. This linear, triatomic pseudohalogen is an easily condensed colorless gas. More commonly encountered in the laboratory is the related compound cyanogen bromide, a room-temperature solid that is widely used in biochemical analysis and preparation.
Contents
Synthesis, basic properties, structure
Although the formula is written CNCl, cyanogen chloride is a molecule with the connectivity ClCN. Carbon and chlorine are linked by a single bond, and carbon and nitrogen by a triple bond. It is a linear molecule, as are the related cyanogen halides (NCF, NCBr, NCI). Cyanogen chloride is produced by the oxidation of sodium cyanide with chlorine. This reaction proceeds via the intermediate cyanogen ((CN)2).[3]
- NaCN + Cl2 → ClCN + NaCl
The compound trimerizes in the presence of acid to the heterocycle called cyanuric chloride.
Cyanogen chloride is slowly hydrolyzed by water to release hydrogen cyanide
- ClCN + H2O → HCN + HOCl
Applications in synthesis
Cyanogen chloride is a precursor to the sulfonyl cyanides[4] and chlorosulfonyl isocyanate, a useful reagent in organic synthesis.[5]
Safety
Also known as CK, cyanogen chloride is a highly toxic blood agent, and was once proposed for use in chemical warfare. It causes immediate injury upon contact with the eyes or respiratory organs. Symptoms of exposure may include drowsiness, rhinorrhea (runny nose), sore throat, coughing, confusion, nausea, vomiting, edema, loss of consciousness, convulsions, paralysis, and death.[6] It is especially dangerous because it is capable of penetrating the filters in gas masks, according to U.S. analysts. CK is unstable due to polymerization, sometimes with explosive violence.[7]
Cyanogen chloride is listed in schedule 3 of the Chemical Weapons Convention: all production must be reported to the OPCW.
References
- ^ Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ http://www.cdc.gov/niosh/ershdb/EmergencyResponseCard_29750039.html
- ^ Coleman, G. H.; Leeper, R. W.; Schulze, C. C. "Cyanogen Chloride" Inorganic Syntheses, 1946, Vol. 2, p. 90.doi:10.1002/9780470132333.ch25
- ^ Vrijland, M. S. A. "Sulfonyl Cyanides: Methanesulfonyl Cyanide" Organic Syntheses, Collected Volume 6, p.727 (1988).
- ^ Graf, R. "Chlorosulfonyl Isocyanate" Organic Syntheses, Collected Volume 5, pages 226ff.
- ^ http://www.bt.cdc.gov/agent/cyanide/erc506-77-4.asp
- ^ FM 3-8 Chemical Reference handbook; US Army; 1967
External links
- NIOSH Emergency Response Card
- eMedicine article
- National Pollutant Inventory – Cyanide compounds fact sheet
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Categories:- Chlorides
- Cyanides
- Inorganic carbon compounds
- Inorganic nitrogen compounds
- Nonmetal halides
- Blood agents
Wikimedia Foundation. 2010.

