- Cyanogen iodide
-
Cyanogen iodide 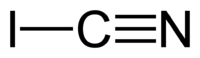

 iodoformonitrileOther namesiodine cyanide, cyanogen iodide
iodoformonitrileOther namesiodine cyanide, cyanogen iodideIdentifiers CAS number 506-78-5 PubChem 10478 ChemSpider 10046 
EC number 208-053-3 RTECS number NN1750000 Jmol-3D images Image 1 - IC#N
Properties Molecular formula ICN Molar mass 152.92 g/mol Appearance white crystals Density 1.84 g/cm3 Melting point 146.7 °C[1]
Solubility in water soluble, slowly reacts Solubility soluble in ethanol, ether Hazards NFPA 704  iodide (verify) (what is:
iodide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Iodine cyanide is the inorganic compound with the formula ICN. It is a pseudohalogen composed of iodine and the cyanide group. It is toxic and occurs as white crystals that react slowly with water to form hydrogen cyanide. It is prepared by combining I2 and sodium cyanide in ice-cold water. The product is extracted with ether.
- I2 + NaCN → NaI + ICN
External links
References
- ^ Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- Langlois M. (1860). "CYANOGÈNE Action de l'iode sur une solution concentrée de cyanure de potassium". Comp Rendus 51: 29. http://gallica.bnf.fr/ark:/12148/bpt6k30082/f29.table.
- Langlois M. (1860). "Ueber die Einwirkung des Jods auf concentrirte Cyankaliumlösung". Annalen der Chemie und Pharmacie 116 (3): 288. doi:10.1002/jlac.18601160303.
Iodine compounds Categories:- Iodine compounds
- Cyanides
- Inorganic compound stubs
Wikimedia Foundation. 2010.

