- Cyanogen bromide
-
Cyanogen bromide 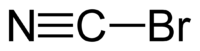
 cyanobromane
cyanobromane
bromidonitridocarbonOther namesBromine cyanide, Bromocyanide, Cyanobromide, Carbononitridic bromide, Bromocyan, Bromocyanogen, Campilit, UN1889, CBIdentifiers CAS number 506-68-3 
PubChem 10476 ChemSpider 10044 
EC number 208-051-2 RTECS number GT2100000 Jmol-3D images Image 1 - BrC#N
Properties Molecular formula CBrN Molar mass 105.92 g mol−1 Appearance Colorless to white solid with pungent odor Density 2.015 g/cm3 Melting point 52 °C, 325 K, 126 °F
Boiling point 61.4 °C, 335 K, 143 °F
Solubility in water Hydrolysis Solubility soluble in alcohol and ether [1] Vapor pressure 13 kPa (20 °C)
16.2 kPa (25 °C)Hazards EU Index Not listed Main hazards Very toxic NFPA 704 U.S. Permissible
exposure limit (PEL)5 mg/m3 Related compounds Related cyanogen halides Cyanogen fluoride
Cyanogen chloride
Cyanogen iodideRelated compounds Cyanogen  bromide (verify) (what is:
bromide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyanogen bromide is a pseudohalogen compound with the formula CNBr. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds.
Contents
Synthesis, basic properties, and structure
Although the formula is most commonly written CNBr, the carbon atom is actually bonded to bromine by a single bond and to nitrogen by a triple bond (i.e. Br–C≡N). The compound is linear and quite polar, but it does not spontaneously ionize in water. Therefore, it dissolves in both water and polar organic solvents.
Cyanogen bromide can be prepared by oxidation of sodium cyanide with bromine, which proceeds in two steps via the intermediate cyanogen ((CN)2 or N≡C–C≡N).
- 2 NaCN + Br2 → (CN)2 + 2 NaBr
- (CN)2 + Br2 → 2 BrCN
Cyanogen bromide is hydrolyzed by water to release hydrogen cyanide and hypobromous acid
- BrCN + H2O → HCN + HOBr
Biochemical applications
The main uses of cyanogen bromide are to immobilize proteins, fragment proteins by cleaving peptide bonds, and synthesize cyanamides and other molecules.
Protein immobilization
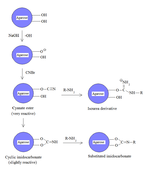
Cyanogen bromide is often used to immobilize proteins by coupling them to reagents such as agarose for affinity chromatography.[2] Because of its simplicity and mild pH conditions, cyanogen bromide activation is the most common method for preparing affinity gels. CNBr is also often used because it reacts with the hydroxyl groups on agarose to form cyanate esters and imidocarbonates. These groups are reacted with primary amines in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding isourea derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.[3]
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a ligand to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak anion exchangers.[3]
Protein cleavage
Cyanogen bromide hydrolyzes peptide bonds at the C-terminus of methionine residues. This reaction is used to reduce the size of polypeptide segments for identification and sequencing.
Mechanism
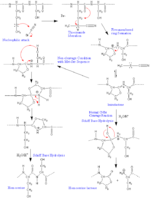
In CNBr, the electron density is shifted away from the carbon atom, making it unusually electrophilic, and towards the more electronegative bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a nucleophile, and the cleavage reaction begins with a nucleophilic acyl substitution reaction in which bromine is ultimately replaced by the sulfur in methionine. This attack is followed by the formation of a five-membered ring as opposed to a six-membered ring, which would entail the formation of a double bond in the ring between nitrogen and carbon. This double bond would result in a rigid ring conformation, thereby destabilizing the molecule. Thus, the five-membered ring is formed so that the double bond is outside the ring, as shown in the figure.
Although the nucleophilic sulfur in methionine is responsible for attacking CNBr, the sulfur in cysteine does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide adduct, leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide nitrogen, which, if attacked by water, would yield cyanic acid and the original cysteine.
Reaction conditions
Cleaving proteins with CNBr requires using a buffer such as 0.1M HCl (hydrochloric acid) or 70% (formic acid).[4] These are the most common buffers for cleavage. An advantage to HCl is that formic acid causes the formation of formyl esters, which complicates protein characterization. However, formic is still often used because it dissolves most proteins. Also, the oxidation of methionine to methionine sulfoxide, which is inert to CNBr attack, occurs more readily in HCl than in formic acid, possibly because formic acid is a reducing acid. Alternative buffers for cleavage include guanidine or urea in HCl because of their ability to unfold proteins, thereby making methionine more accessible to CNBr.[5]
Note that water is required for normal peptide bond cleavage of the iminolactone intermediate. In formic acid, cleavage of Met-Ser and Met-Thr bonds is enhanced with increased water concentration because these conditions favor the addition of water across the imine rather than reaction of the side chain hydroxyl with the imine. Lowered pH tends to increase cleavage rates by inhibiting methionine side chain oxidation.[5]
Side reactions
When methionine is followed by serine or threonine, side reactions can occur that destroy the methionine without peptide bond cleavage. Normally, once the iminolactone is formed (refer to figure), water and acid can react with the imine to cleave the peptide bond, forming a homoserine lactone and new C-terminal peptide. However, if the adjacent amino acid to methionine has a hydroxyl or sulfhydryl group, this group can react with the imine to form a homoserine without peptide bond cleavage.[5] These two cases are shown in the figure.
Organic synthesis
Cyanogen bromide is also widely used in organic synthesis. As stated earlier, the reagent is prone to attack by nucleophiles such as amines and alcohols because of the electrophilic carbon. In the synthesis of cyanamides and dicyanamides, primary and secondary amines react with CNBr to yield mono- and dialkylcyanamides, which can further react with amines and hydroxylamine to yield guanidines and hydroxyguanidines. In the von Braun reaction, tertiary amines react with CNBr to yield disubstituted cyanamides and an alkyl bromide. CNBr can be used to prepare aryl nitriles, nitriles, anhydrides, and cyanates. It can also serve as a cleaving agent.[6]
Toxicity, storage, and deactivation
Cyanogen bromide is moisture-sensitive but can be stored under dry conditions at 2 to 8 °C for extended periods of time.[3]
Cyanogen bromide is volatile, and readily absorbed through the skin or gastrointestinal tract. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of nonspecific symptoms. Exposure to even small amounts may cause convulsions or death. LD50 orally in rats is reported as 25–50 mg/kg.[7]
To deactivate CNBr in a solution not exceeding 60 g/L of CNBr (dilute if necessary), the recommended method is to add 1 mol/L NaOH and 1 mol/L NaOCl in volumes of ratio 1:1:2 (CNBr solution:NaOH:NaOCl).[8] The aqueous alkali hydroxide instantly hydrolyzes CNBr to alkali cyanide and bromide. The cyanide can then be oxidized by sodium or calcium hypochlorite to the less toxic cyanate ion. Note that deactivation is extremely exothermic and may be explosive.[7]
See also
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ^ Immobilized Affinity Ligand Techniques. Greg T. Hermanson, A. Krishna Mallia and Paul K. Smith. Academic Press, © 1992.
- ^ a b c "Cyanogen Bromide Activated Matrices." Sigma Product Information (Sigma).
- ^ Schroeder, W.A., Joan Balog Shelton, and J. Roger Shelton (1969). "An Examination of Conditions for the Cleavage of Polypeptide Chains with Cyanogen Bromide." Archives of Biochemistry and Biophysics 130 (1): 551-6.
- ^ a b c Kaiser, Raymond and Lorraine Metzka (1999). "Enhancement of Cyanogen Bromide Cleavage Yields for Methionyl-Serine and Methionyl-Threonine Peptide Bonds." Analytical Biochemistry 266 (1): 1-8.
- ^ Kumar, Vinod (2005)."Cyanogen Bromide (CNBr)." Thieme 10 (1): 1638-9.
- ^ a b "Cyanogen Bromide." NIH Division of Occupational Health and Safety.
- ^ Lunn, George and Eric B. Sansone (1985). "Destruction of Cyanogen Bromide and Inorganic Cyanides". Analytical Biochemistry 147: 245. doi:10.1016/0003-2697(85)90034-X. PMID 4025821.
Further reading
- Erhard Gross; Bernhard Witkop (June 1, 1962). "Nonenzymatic Cleavage of Peptide Bonds: The Methionine Residues in Bovine Pancreatic Ribonuclease.". Journal of Biological Chemistry 237 (6): 1856–60. PMID 13902203. http://www.jbc.org/cgi/reprint/237/6/1856.
- A.S. Inglis; P. Edman, A; Edman, P (1970). "Mechanism of Cyanogen Bromide Reaction with Methionine in Peptides and Proteins.". Analytical Biochemistry 37 (1): 73–80. doi:10.1016/0003-2697(70)90259-9. PMID 5506566.
External links
Categories:- Bromides
- Cyanides
- Inorganic nitrogen compounds
- Nonmetal halides
Wikimedia Foundation. 2010.