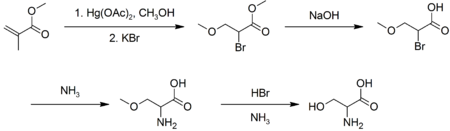- Serine
-
Serine 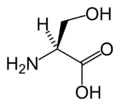
 SerineOther names2-Amino-3-hydroxypropanoic acid
SerineOther names2-Amino-3-hydroxypropanoic acidIdentifiers CAS number 56-45-1  , 302-84-1
, 302-84-1  , 312-84-5
, 312-84-5 
PubChem 617 ChemSpider 5736 (L-form)  , 597
, 597UNII 452VLY9402 
EC-number 206-130-6 DrugBank DB00133 ChEBI CHEBI:17115 
ChEMBL CHEMBL11298 
IUPHAR ligand 726 Jmol-3D images Image 1 - C([C@@H](C(=O)O)N)O
Properties[2] Molecular formula C3H7NO3 Molar mass 105.09 g mol−1 Appearance white crystals or powder Density 1.603 g/cm3 (22 ºC) Melting point 246 ºC decomp.
Solubility in water soluble Acidity (pKa) 2.21 (carboxyl), 9.15 (amino)[1] Supplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Serine (abbreviated as Ser or S)[3] is an amino acid with the formula HO2CCH(NH2)CH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.
Contents
Occurrence and biosynthesis
This compound is one of the naturally occurring proteinogenic amino acids. Its codons are UCU, UCC, UCA, UCG, AGU and AGC. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865. Its name is derived from the Latin for silk, sericum. Serine's structure was established in 1902.[citation needed]
The biosynthesis of serine starts with the oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate and NADH. Reductive amination of this ketone followed by hydrolysis gives serine. Serine hydroxymethyltransferase catalyzes the reversible, simultaneous conversions of L-serine to glycine (retro-aldol cleavage) and 5,6,7,8-tetrahydrofolate to 5,10-methylenetetrahydrofolate (hydrolysis).[4]
This compound may also be naturally produced when UV light illuminates simple ices such as a combination of water, methanol, hydrogen cyanide, and ammonia, suggesting that it may be easily produced in cold regions of space.[5]
Production
Industrially, L-serine is produced by fermentation, with an estimated 100-1000 tonnes per year produced.[6] In the laboratory, racemic serine can be prepared from methyl acrylate via several steps:[7]
Biological function
Metabolic
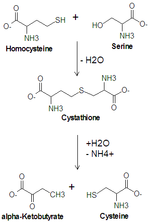 Cysteine synthesis from serine. Cystathionine beta synthase catalyzes the upper reaction and cystathionine gamma-lyase catalyzes the lower reaction.
Cysteine synthesis from serine. Cystathionine beta synthase catalyzes the upper reaction and cystathionine gamma-lyase catalyzes the lower reaction.
Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines. It is the precursor to several amino acids including glycine and cysteine, and tryptophan in bacteria. It is also the precursor to numerous other metabolites, including sphingolipids and folate, which is the principal donor of one-carbon fragments in biosynthesis.
Structural role
Serine plays an important role in the catalytic function of many enzymes. It has been shown to occur in the active sites of chymotrypsin, trypsin, and many other enzymes. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely.
As a constituent (residue) of proteins, its side chain can undergo O-linked glycosylation, which may be functionally related to[clarification needed] diabetes.
It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes. Phosphorylated serine residues are often referred to as phosphoserine.
Serine proteases are a common type of protease.
Signaling
D-Serine, synthesized in the brain by serine racemase from L-serine (its enantiomer), serves as both a neurotransmitter and a gliotransmitter by activating NMDA receptors, making them able to open if they then also bind glutamate. D-serine is a potent agonist at the glycine site of the NMDA-type glutamate receptor. For the receptor to open, glutamate and either glycine or D-serine must bind to it. In fact, D-serine is a more potent agonist at the glycine site on the NMDAR than glycine itself. D-serine was only thought to exist in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signalling molecule in the brain, soon after the discovery of D-aspartate. Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site. [8]
See also
- Serine aggregation properties in Serine octamer clusters
References
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-512. ISBN 0-8493-0462-8..
- ^ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem. 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ^ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000), Principles of Biochemistry (3rd ed.), New York: W. H. Freeman, ISBN 1-57259-153-6.
- ^ Elsila, Jamie E.; Dworkin, Jason P.; Bernstein, Max P.; Martin, Mildred P.; Sandford, Scott A. (2007), "Mechanisms of Amino Acid Formation in Interstellar Ice Analogs", Astrophys. J. 660 (1): 911–18, doi:10.1086/513141
- ^ Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker (2005), "Amino Acids", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a02_057.pub2
- ^ Carter, Herbert E.; West, Harold D. (1940), "dl-Serine", Org. Synth. 20: 81, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0774; Coll. Vol. 3: 774.
- ^ Mothet, Jean-Pierre; Parent, Angèle T.; Wolosker, Herman; Brady, Roscoe O., Jr.; Linden, David J.; Ferris, Christopher D.; Rogawski, Michael A.; Snyder, Solomon H. (2000), "d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor", Proc. Natl. Acad. Sci. USA 97 (9): 4926–31, doi:10.1073/pnas.97.9.4926, PMC 18334, PMID 10781100, http://www.pnas.org/cgi/pmidlookup?view=long&pmid=10781100.
The 20 common amino acids By properties AliphaticBranched-chain amino acids (Valine · Isoleucine · Leucine) · Methionine · Alanine · Proline · GlycineAromaticPolar, unchargedPositive charge (pKa)Negative charge (pKa)GeneralOther classifications biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i K→acetyl-CoA G glycine→serine→otherα-Ketoisovaleric acid · Isobutyryl-CoA · Methacrylyl-CoA · 3-Hydroxyisobutyryl-CoA · 3-Hydroxyisobutyric acid · 2-Methyl-3-oxopropanoic acidG→fumarateG→oxaloacetatesee urea cycleOther biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iNeurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
Glycinergics Receptor
ligandsAgonistsAlanine • Cycloserine • Dimethylglycine • Glycine • Hypotaurine • Methylglycine (Sarcosine) • Milacemide • Serine • Taurine • Trimethylglycine (Betaine)Reuptake
inhibitorsPlasmalemmalGlyT1 inhibitorsGlyT2 inhibitorsVIAAT InhibitorsEnzyme
inhibitorsSHMT InhibitorsGDC InhibitorsDAAO InhibitorsOthers 3-Phosphoglyceric acid • SerineCategories:- Proteinogenic amino acids
- Glucogenic amino acids
Wikimedia Foundation. 2010.