- Adenosine monophosphate
-
Adenosine monophosphate 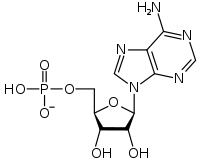
 5'-Adenylic acid
5'-Adenylic acidIdentifiers CAS number 61-19-8 
ChemSpider 5858 
UNII 415SHH325A 
DrugBank DB00131 KEGG C00020 
MeSH Adenosine+monophosphate ChEBI CHEBI:16027 
ChEMBL CHEMBL752 
IUPHAR ligand 2455 Jmol-3D images Image 1
Image 2- O=P(O)(O)OC[C@H]3O[C@@H](n2cnc1c(ncnc12)N)[C@H](O)[C@@H]3O
c1nc(c2c(n1)n(cn2)[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(O)O)O)O)N
- InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1

Key: UDMBCSSLTHHNCD-KQYNXXCUSA-N
InChI=1/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1
Key: UDMBCSSLTHHNCD-KQYNXXCUBP
Properties Molecular formula C10H14N5O7P Molar mass 347.22 g/mol Acidity (pKa) 0.9, 3.8, 6.1  monophosphate (verify) (what is:
monophosphate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine. As a substituent it takes the form of the prefix adenylyl-.
Contents
Production and degradation
AMP can be produced during ATP synthesis by the enzyme adenylate kinase by combining two ADP molecules:
- 2 ADP → ATP + AMP
Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP:
- ADP → AMP + Pi
AMP can also be formed by hydrolysis of ATP into AMP and pyrophosphate:
- ATP → AMP + PPi
When RNA is broken down by living systems, nucleoside monophosphates, including adenosine monophosphate, are formed.
AMP can be regenerated to ATP as follows:
- AMP + ATP → 2 ADP (adenylate kinase in the opposite direction)
- ADP + Pi → ATP (this step is most often performed in aerobes by the ATP synthase during oxidative phosphorylation)
AMP can be converted into IMP by the enzyme myoadenylate deaminase, freeing an ammonia group.
In a catabolic pathway, adenosine monophosphate can be converted to uric acid, which is excreted from the body.
cAMP
AMP can also exist as a cyclic structure known as cyclic AMP (or cAMP). Within certain cells the enzyme adenylate cyclase makes cAMP from ATP, and typically this reaction is regulated by hormones such as adrenaline or glucagon. cAMP plays an important role in intracellular signaling.
See also
References
External links
- Ming D, Ninomiya Y, Margolskee RF (1999). "Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds". Proc Natl Acad Sci 96 (17): 9903–9908. doi:10.1073/pnas.96.17.9903. PMC 22308. PMID 10449792. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=22308.
Nucleic acid constituents Nucleobase Nucleoside Nucleotide
(Nucleoside monophosphate)Nucleoside diphosphate Nucleoside triphosphate biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iNeurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Nucleotides
- Purines
- O=P(O)(O)OC[C@H]3O[C@@H](n2cnc1c(ncnc12)N)[C@H](O)[C@@H]3O
Wikimedia Foundation. 2010.
