- Deoxyadenosine
-
Deoxyadenosine 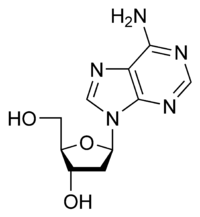
Identifiers CAS number 958-09-8 
PubChem 636 ChemSpider 13135 
UNII P582C98ULC 
MeSH 2'-deoxyadenosine ChEBI CHEBI:17256 
ChEMBL CHEMBL416340 
Jmol-3D images Image 1 - n2c1c(ncnc1n(c2)[C@@H]3O[C@@H]([C@@H](O)C3)CO)N
- InChI=1S/C10H13N5O3/c11-9-8-10(13-3-12-9)15(4-14-8)7-1-5(17)6(2-16)18-7/h3-7,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,7+/m0/s1

Key: OLXZPDWKRNYJJZ-RRKCRQDMSA-N
InChI=1/C10H13N5O3/c11-9-8-10(13-3-12-9)15(4-14-8)7-1-5(17)6(2-16)18-7/h3-7,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,7+/m0/s1
Key: OLXZPDWKRNYJJZ-RRKCRQDMBK
Properties Molecular formula C10H13N5O3 Molar mass 251.24192  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Deoxyadenosine is a deoxyribonucleoside. It is a derivative of the nucleoside adenosine, differing from the latter by the replacement of a hydroxyl group (-OH) by hydrogen (-H) at the 2' position of its ribose sugar moiety. Deoxyadenosine is the DNA nucleoside A, which pairs with deoxythymidine (T) in double-stranded DNA.
See also
- Deoxyribonucleotide
- Cordycepin (3'-deoxyadenosine)
References
Nucleic acid constituents Nucleobase Nucleoside Nucleotide
(Nucleoside monophosphate)Nucleoside diphosphate Nucleoside triphosphate biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Biochemistry stubs
- Nucleosides
- Purines
Wikimedia Foundation. 2010.
