- Ribose
-
D-Ribose 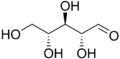

 D-Ribose
D-RiboseIdentifiers CAS number 50-69-1 
PubChem 5779 EC-number 200-059-4 DrugBank DB01936 ChEMBL CHEMBL1159662 
Properties[1][2] Molecular formula C5H10O5 Molar mass 150.13 g/mol Appearance white solid Melting point 95 °C, 368 K, 203 °F
Solubility in water very soluble Chiral rotation [α]D −21.5º (H2O) Related compounds Related aldopentoses Arabinose
Xylose
LyxoseRelated compounds Deoxyribose  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide (simple sugar) with linear form H–(C=O)–(CHOH)4–H, which has all the hydroxyl groups on the same side in the Fischer projection.
The term may refer to any of two enantiomers: almost always to D-ribose, which occurs widely in nature and is discussed here; or to its synthetic mirror image L-ribose, which is not found in nature and is of limited interest.
D-Ribose was first reported in 1891 by Emil Fischer. It is a C'-2 carbon enantiomer of the sugar D-arabinose (both isomers of which are named for their source, gum arabic) and ribose itself is named as a transposition of the name of arabinose.[3]
Ribose comprises the backbone of RNA, a biopolymer that is the basis of genetic transcription. It is related to deoxyribose, as found in DNA. Once phosphorylated, ribose can become a subunit of ATP, NADH, and several other compounds that are critical to metabolism like the secondary messengers cAMP and cGMP.
Structure
Ribose is an aldopentose, that is a monosaccharide containing five carbon atoms that, in its open chain form, has an aldehyde functional group at one end. In the conventional numbering scheme for monosaccharides, the carbon atoms are numbered from C1' (in the aldehyde group) to C5'. The deoxyribose derivative, found in DNA, differs from ribose by having a hydrogen atom in place of the hydroxyl group in carbon C2'.
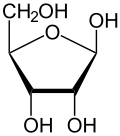
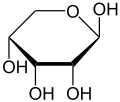
Like many monosaccharides, ribose occurs in water as the linear form H–(C=O)–(CHOH)4–H and any of two ring forms: ribofuranose ("C3'-endo"), with a five-membered ring, and ribopyranose ("C2'-endo"), with a six-membered ring. The ribofuranose form is predominant in aqueous solution.
The "D-" in the name D-ribose refers to the stereochemistry of the chiral carbon atom farthest away from the aldehyde group (C4'). In D-ribose, as in all D-sugars, this carbon atom has the same configuration as in D-glyceraldehyde.
Phosphorylation
In biology, D-ribose must be phosphorylated by the cell before it can be used. Ribokinase catalyzes this reaction by converting D-ribose to D-ribose 5-phosphate. Once converted, D-ribose-5-phosphate is available for the manufacturing of the amino acids tryptophan and histidine, or for use in the pentose phosphate pathway. The absorption of D-ribose is 88–100% in the small intestines (up to 200 mg/kg/hr).[4]
References
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 8205.
- ^ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-506. ISBN 0-8493-0462-8..
- ^ Nechamkin, Howard (1958). "Some interesting etymological derivations of chemical terminology". Science Education 42: 463. doi:10.1002/sce.3730420523.
- ^ [1][dead link]
Types of carbohydrates General: Geometry Monosaccharides Aldodiose (Glycolaldehyde)Ketopentose (Ribulose, Xylulose)
Aldopentose (Ribose, Arabinose, Xylose, Lyxose)
Deoxy sugar (Deoxyribose)>7Multiple Other oligosaccharidesGlucose/Glucan: Glycogen · Starch (Amylose, Amylopectin) · Cellulose · Dextrin/Dextran · Beta-glucan (Zymosan, Lentinan, Sizofiran) · Maltodextrin
Fructose/Fructan: Inulin · Levan beta 2→6
N-Acetylglucosamine: Chitinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Aldopentoses
Wikimedia Foundation. 2010.
