- Galactose
-
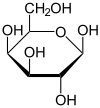
Galactose 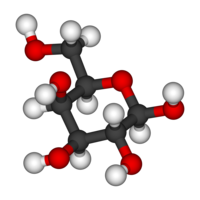

Identifiers CAS number 26566-61-0 
PubChem 439357 ChemSpider 388480 
UNII X2RN3Q8DNE 
KEGG D04291 
MeSH Galactose ChEBI CHEBI:28061 
ChEMBL CHEMBL300520 
Jmol-3D images Image 1 - O[C@H]1[C@@H](O)[C@H](O[C@H](O)[C@@H]1O)CO
Properties Molecular formula C6H12O6 Molar mass 180.156 g mol−1 Melting point 167°C
Solubility in water 683.0 g/L  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Galactose (from Greek γάλακτος galaktos "milk"), sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose.
Galactan is a polymer of the sugar galactose found in hemicellulose. Galactan can be converted to galactose by hydrolysis.
Contents
Structure and isomerism
Galactose exists in both open-chain and cyclic form. The open-chain form has a carbonyl at the end of the chain.
Four isomers are cyclic, two of them with a pyranose (six-membered) ring, two with a furanose (five-membered) ring. Galactofuranose occurs in bacteria, fungi and protozoa. [1]
Relationship to lactose
Galactose is a monosaccharide. When combined with glucose (monosacccharide), through a dehydration reaction, the result is the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and β-galactosidase. The latter is produced by the lac operon in Escherichia coli.
Lactose is found primarily in milk and milk products. Galactose metabolism, which converts galactose into glucose, is carried out by the three principal enzymes in a mechanism known as the Leloir pathway. The enzymes are listed in the order of the metabolic pathway: galactokinase (GALK), galactose-1-phosphate uridyltransferase (GALT), and UDP-galactose-4’-epimerase (GALE).
In the human body, glucose is changed into galactose via hexoneogenesis to enable the mammary glands to secrete lactose. However, most galactose in breast milk is synthesized from galactose taken up from the blood, and only 35±6% is made by de novo synthesis. [2] Glycerol also contributes some to the mammary galactose production.[3]
Galactose metabolism
Glucose is the primary metabolic fuel for humans. It is more stable than galactose and is less susceptible to the formation of nonspecific glycoconjugates, molecules with at least one sugar attached to a protein or lipid. Many speculate that it is for this reason that a pathway for rapid conversion from galactose to glucose has been highly conserved among many species.[4]
The main pathway of galactose metabolism is the Leloir pathway; humans and other species, however, have been noted to contain several alternate pathways, such as the De Ley Doudoroff pathway. The Leloir pathway consists of the latter stage of a two-part process that converts β-D-galactose to UDP-glucose. The initial stage is the conversion of β-D-galactose to α-D-galactose by the enzyme, mutarotase (GALM). The Leloir pathway then carries out the conversion of α-D-galactose to UDP-glucose via three principle enzymes. Galactokinase (GALK) phosphorylates α-D-galactose to galactose-1-phosphate, or Gal-1-P. Galactose-1-phosphate uridyltransferase (GALT) then transfers a UMP group from UDP-glucose to Gal-1-P to form UDP-galactose. Finally, UDP galactose-4’-epimerase (GALE) interconverts UDP-galactose and UDP-glucose, thereby completing the pathway.[5]

Sources
Galactose is found in dairy products, sugar beets, and other gums and mucilages. It is also synthesized by the body, where it forms part of glycolipids and glycoproteins in several tissues.
Clinical significance
Chronic systemic exposure of mice, rats, and Drosophila to D-galactose causes the acceleration of senescence and has been used as an aging model.[6] Two studies have suggested a possible link between galactose in milk and ovarian cancer.[7][8] Other studies show no correlation, even in the presence of defective galactose metabolism.[9][10] More recently, pooled analysis done by the Harvard School of Public Health showed no specific correlation between lactose-containing foods and ovarian cancer, and showed statistically insignificant increases in risk for consumption of lactose at ≥30 g/d.[11] More research is necessary to ascertain possible risks.
Some ongoing studies suggest galactose may have a role in treatment of focal segmental glomerulosclerosis (a kidney disease resulting in kidney failure and proteinuria).[citation needed] This effect is likely to be a result of binding of galactose to FSGS factor.[citation needed]
Galactose is a component of the antigens present on blood cells that determine blood type within the ABO blood group system.[12]
See also
- Galactolysis
References
- ^ Nassau et al. Galactofuranose Biosynthesis in Escherichia coli K-12:... JOURNAL OF BACTERIOLOGY, Feb. 1996, p. 1047–1052
- ^ Sunehag A, Tigas S, Haymond MW (January 2003). "Contribution of plasma galactose and glucose to milk lactose synthesis during galactose ingestion". J. Clin. Endocrinol. Metab. 88 (1): 225–9. doi:10.1210/jc.2002-020768. PMID 12519857. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=12519857.
- ^ Sunehag AL, Louie K, Bier JL, Tigas S, Haymond MW (January 2002). "Hexoneogenesis in the human breast during lactation". J. Clin. Endocrinol. Metab. 87 (1): 297–301. doi:10.1210/jc.87.1.297. PMID 11788663. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=11788663.
- ^ Fridovich-Keil JL, Walter JH. "Galactosemia". The Online Metabolic and Molecular Bases of Inherited Disease. http://www.ommbid.com/OMMBID/the_online_metabolic_and_molecular_bases_of_inherited_disease/b/abstract/part7/ch72.
a 4 b 21 c 22 d 22 - ^ Bosch AM (August 2006). "Classical galactosaemia revisited". J. Inherit. Metab. Dis. 29 (4): 516–25. doi:10.1007/s10545-006-0382-0. PMID 16838075.
a 517 b 516 c 519 - ^ Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. (2006). "Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid". Journal of neuroscience research 84 (3): 647–654. doi:10.1002/jnr.20899. PMID 16710848.
- ^ Cramer D (1989). "Lactase persistence and milk consumption as determinants of ovarian cancer risk". Am J Epidemiol 130 (5): 904–10. PMID 2510499.
- ^ Cramer D, Harlow B, Willett W, Welch W, Bell D, Scully R, Ng W, Knapp R (1989). "Galactose consumption and metabolism in relation to the risk of ovarian cancer". Lancet 2 (8654): 66–71. doi:10.1016/S0140-6736(89)90313-9. PMID 2567871.
- ^ Marc T. Goodman , Anna H. Wu , Ko-Hui Tung , Katharine McDuffie , Daniel W. Cramer , Lynne R. Wilkens , Keith Terada , Juergen K. V. Reichardt , and Won G. Ng (2002). "Association of Galactose-1-Phosphate Uridyltransferase Activity and N314D Genotype with the Risk of Ovarian Cancer". Am. J. Epidemiol 156 (8): 693–701. doi:10.1093/aje/kwf104. PMID 12370157.
- ^ Fung, W. L. Alan, Risch, Harvey, McLaughlin, John, Rosen, Barry, Cole, David, Vesprini, Danny, Narod, Steven A. (2003). "The N314D Polymorphism of Galactose-1-Phosphate Uridyl Transferase Does Not Modify the Risk of Ovarian Cancer". Cancer Epidemiol Biomarkers Prev 12 (7): 678–80. PMID 12869412.
- ^ Genkinger, Jeanine M., Hunter, David J., Spiegelman, Donna, Anderson, Kristin E., Arslan, Alan, Beeson, W. Lawrence, Buring, Julie E., Fraser, Gary E., Freudenheim, Jo L., Goldbohm, R. Alexandra, Hankinson, Susan E., Jacobs, David R., Jr., Koushik, Anita, Lacey, James V., Jr., Larsson, Susanna C., Leitzmann, Michael, McCullough, Marji L., Miller, Anthony B., Rodriguez, Carmen, Rohan, Thomas E., Schouten, Leo J., Shore, Roy, Smit, Ellen, Wolk, Alicja, Zhang, Shumin M., Smith-Warner, Stephanie A. (2006). "Dairy Products and Ovarian Cancer: A Pooled Analysis of 12 Cohort Studies". Cancer Epidemiol Biomarkers Prev 15 (2): 364–372. doi:10.1158/1055-9965.EPI-05-0484. PMID 16492930.
- ^ Peter H. Raven & George B. Johnson (1995). Carol J. Mills (ed). ed. Understanding Biology (3rd ed.). WM C. Brown. pp. 203. ISBN 0-697-22213-6.
Types of carbohydrates General: Geometry Monosaccharides Aldodiose (Glycolaldehyde)Ketopentose (Ribulose, Xylulose)
Aldopentose (Ribose, Arabinose, Xylose, Lyxose)
Deoxy sugar (Deoxyribose)Ketoheptose (Sedoheptulose, Mannoheptulose)>7Multiple Other oligosaccharidesGlucose/Glucan: Glycogen · Starch (Amylose, Amylopectin) · Cellulose · Dextrin/Dextran · Beta-glucan (Zymosan, Lentinan, Sizofiran) · Maltodextrin
Fructose/Fructan: Inulin · Levan beta 2→6
Galactose/Galactan
N-Acetylglucosamine: Chitinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i
Wikimedia Foundation. 2010.

