- Glycoprotein
-
Not to be confused with peptidoglycan or proteoglycan.
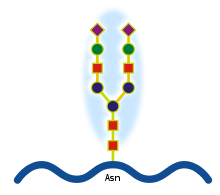 N-linked protein glycosylation (N-glycosylation of N-glycans) at Asn residues (Asn-x-Ser/Thr motifs) in glycoproteins.[1]
N-linked protein glycosylation (N-glycosylation of N-glycans) at Asn residues (Asn-x-Ser/Thr motifs) in glycoproteins.[1]
Glycoproteins are proteins that contain oligosaccharide chains (glycans) covalently attached to polypeptide side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. In proteins that have segments extending extracellularly, the extracellular segments are often glycosylated. Glycoproteins are often important integral membrane proteins, where they play a role in cell–cell interactions. Glycoproteins also occur in the cytosol, but their functions and the pathways producing these modifications in this compartment are less well-understood.[2]
Contents
N-glycosylation and O-glycosylation
There are two types of glycoproteins:
- In N-glycosylation (see on the right), the addition of sugar chains can happen at the amide nitrogen on the side-chain of the asparagine.
- In O-glycosylation, the addition of sugar chains can happen on the hydroxyl oxygen on the side-chain of hydroxylysine, hydroxyproline, serine, or threonine.
Monosaccharides
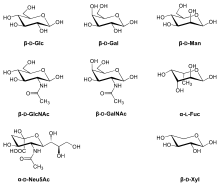
Monosaccharides commonly found in eukaryotic glycoproteins include:[3]
The principal sugars found in human glycoproteins[4] Sugar Type Abbreviation β-D-Glucose Hexose Glc β-D-Galactose Hexose Gal β-D-Mannose Hexose Man α-L-Fucose Deoxyhexose Fuc N-Acetylgalactosamine Aminohexose GalNAc N-Acetylglucosamine Aminohexose GlcNAc N-Acetylneuraminic acid Aminononulosonic acid
(Sialic acid)NeuNAc Xylose Pentose Xyl The sugar group(s) can assist in protein folding or improve proteins' stability.
Examples
One example of glycoproteins found in the body is mucins, which are secreted in the mucus of the respiratory and digestive tracts. The sugars attached to mucins give them considerable water-holding capacity and also make them resistant to proteolysis by digestive enzymes.
Glycoproteins are important for white blood cell recognition, especially in mammals.[citation needed] Examples of glycoproteins in the immune system are:
- molecules such as antibodies (immunoglobulins), which interact directly with antigens.
- molecules of the major histocompatibility complex (or MHC), which are expressed on the surface of cells and interact with T cells as part of the adaptive immune response.
Other examples of glycoproteins include:
- glycoprotein IIb/IIIa, an integrin found on platelets that is required for normal platelet aggregation and adherence to the endothelium.
- components of the zona pellucida, which surrounds the oocyte, and is important for sperm-egg interaction.
- structural glycoproteins, which occur in connective tissue. These help bind together the fibers, cells, and ground substance of connective tissue. They may also help components of the tissue bind to inorganic substances, such as calcium in bone.
- Glycoprotein-41 (gp41) and glycoprotein-120 (gp120) are HIV viral coat proteins.
Soluble glycoproteins often show a high viscosity, for example, in egg white and blood plasma.
- Miraculin, which alters human tongue receptors to recognize sour foods as sweet.
Hormones
Hormones that are glycoproteins include:
- Follicle-stimulating hormone
- Luteinizing hormone
- Thyroid-stimulating hormone
- Human chorionic gonadotropin
- Alpha-fetoprotein
- Erythropoietin (EPO)
Functions
Some functions served by glycoproteins[5] Function Glycoproteins Structural molecule Collagens Lubricant and protective agent Mucins Transport molecule Transferrin, ceruloplasmin Immunologic molecule Immunoglobins, histocompatibility antigens Hormone Human chorionic gonadotropin (HCG), thyroid-stimulating hormone (TSH) Enzyme Various, e.g., alkaline phosphatase Cell attachment-recognition site Various proteins involved in cell–cell (e.g., sperm–oocyte), virus–cell, bacterium–cell, and hormone–cell interactions Antifreeze protein Certain plasma proteins of coldwater fish Interact with specific carbohydrates Lectins, selectins (cell adhesion lectins), antibodies Receptor Various proteins involved in hormone and drug action Affect folding of certain proteins Calnexin, calreticulin Regulation of development Notch and its analogs, key proteins in development Hemostasis (and thrombosis) Specific glycoproteins on the surface membranes of platelets Analysis
A variety of methods used in detection, purification, and structural analysis of glycoproteins are[6][7]
Some important methods used to study glycoproteins Method Use Periodic acid-Schiff stain Detects glycoproteins as pink bands after electrophoretic separation. Incubation of cultured cells with glycoproteins as radioactive decay bands Leads to detection of a radioactive sugar after electrophoretic separation. Treatment with appropriate endo- or exoglycosidase or phospholipases Resultant shifts in electrophoretic migration help distinguish among proteins with N-glycan, O-glycan, or GPI linkages and also between high mannose and complex N-glycans. Agarose-lectin column chromatography, lectin affinity chromatography To purify glycoproteins or glycopeptides that bind the particular lectin used. Lectin affinity electrophoresis Resultant shifts in electrophoretic migration help distinguish and characterize glycoforms, i.e. variants of a glycoprotein differing in carbohydrate. Compositional analysis following acid hydrolysis Identifies sugars that the glycoprotein contains and their stoichiometry. Mass spectrometry Provides information on molecular mass, composition, sequence, and sometimes branching of a glycan chain. NMR spectroscopy To identify specific sugars, their sequence, linkages, and the anomeric nature of glycosidic chain. Dual Polarisation Interferometry Measures the mechanisms underlying the biomolecular interactions, including reaction rates, affinities and associated conformational changes. Methylation (linkage) analysis To determine linkage between sugars. Amino acid or cDNA sequencing Determination of amino acid sequence. See also
- P-glycoprotein
- Glycopeptide
- Proteoglycan
- Glycocalyx
- Ero1
- Gp120
- Gp41
- Miraculin
- Female Sperm Storage
References
- ^ Ruddock & Molinari (2006) Journal of Cell Science 119, 4373–4380
- ^ Funakoshi Y, Suzuki T (January 2009). "Glycobiology in the cytosol: The bitter side of a sweet world". Biochim. Biophys. Acta 1790 (2): 81–94. doi:10.1016/j.bbagen.2008.09.009. PMID 18952151.
- ^ Robert K. Murray, Daryl K. Granner & Victor W. Rodwell: "Harper's Illustrated Biochemistry 27th Ed.", p. 526, McGraw-Hill, 2006
- ^ https://www.sigmaaldrich.com/img/assets/15880/glycan_classification.pdf
- ^ Ibid., p. 524
- ^ Ibid., p. 525
- ^ Anne Dell, Howard R Morris: "Glycoprotein structure determination by mass spectrometry", Science 291(5512), 2351–2356 (2001), Review
External links
- Structure of Glycoprotein and Carbohydrate Chain – Home Page for Learning Environmental Chemistry
- Biochemistry 5thE 11.3. Carbohydrates Can Be Attached to Proteins to Form Glycoproteins
- Carbohydrate Chemistry and Glycobiology: A Web Tour SPECIAL WeB SUPPLEMENT Science 23 March 2001 Vol 291, Issue 5512, Pages 2263–2502
- MeSH Glycoproteins
Mucoproteins OtherProteoglycan Testican · PerlecanChondroitin sulfate proteoglycans: Aggrecan · Neurocan · Brevican · CD44 · CSPG4 · CSPG5 · Platelet factor 4 · Structural maintenance of chromosomes 3Fibromodulin · Lumican · KeratocanOther Activin and inhibin · ADAM · Alpha 1-antichymotrypsin · Apolipoprotein H · CD70 · Asialoglycoprotein · Avidin · B-cell activating factor · 4-1BB ligand · Cholesterylester transfer protein · Clusterin · Colony-stimulating factor · Hemopexin · Lactoferrin · Membrane glycoproteins · Myelin protein zero · Osteonectin · Protein C · Protein S · Serum amyloid P component · Sialoglycoprotein (CD43, Glycophorin, Glycophorin C) · Thrombopoietin · Thyroglobulin · Thyroxine-binding proteins · Transcortin · Tumor necrosis factor-alpha · Uteroglobin · Vitronectinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iAnabolism Catabolism Neuraminidase · Beta-galactosidase · Hexosaminidase · mannosidase (alpha-Mannosidase, beta-mannosidase) · Aspartylglucosaminidase · Fucosidase · NAGATransport M6P tagging (LSD) Inborn error of carbohydrate metabolism: glycoproteinosis (E77, 271.8) Anabolism Post-translational modification
of lysosomal enzymesCatabolism Aspartylglucosaminuria · Fucosidosis · mannosidosis (Alpha-mannosidosis, Beta-mannosidosis) · Sialidosis · Schindler diseaseOther Categories:- Glycoproteins
- Carbohydrate chemistry
Wikimedia Foundation. 2010.