- Erythropoietin
-
Erythropoietin, or its alternatives erythropoetin or erithropoyetin (
 /ɨˌrɪθrɵˈpɔɪ.ɨtɨn/, /ɨˌrɪθrɵˈpɔɪtən/, or /ɨˌriːθrɵ-/) or EPO, is a glycoprotein hormone that controls erythropoiesis, or red blood cell production. It is a cytokine for erythrocyte (red blood cell) precursors in the bone marrow.
/ɨˌrɪθrɵˈpɔɪ.ɨtɨn/, /ɨˌrɪθrɵˈpɔɪtən/, or /ɨˌriːθrɵ-/) or EPO, is a glycoprotein hormone that controls erythropoiesis, or red blood cell production. It is a cytokine for erythrocyte (red blood cell) precursors in the bone marrow.Also called hematopoietin or hemopoietin, it is produced by interstitial fibroblasts in the kidney in close association with peritubular capillary and tubular epithelial cells. It is also produced in perisinusoidal Ito cells in the liver. While liver production predominates in the fetal and perinatal period, renal production is predominant during adulthood. Erythropoietin is the hormone that regulates red blood cell production. It also has other known biological functions. For example, erythropoietin plays an important role in the brain's response to neuronal injury.[1] EPO is also involved in the wound healing process.[2]
When exogenous EPO is used as a performance-enhancing drug, it is classified as an erythropoiesis-stimulating agent (ESA). Exogenous EPO can often be detected in blood, due to slight difference from the endogenous protein, for example in features of posttranslational modification.
Contents
History
In 1906, Paul Carnot, a professor of medicine in Paris,France, and his assistant DeFlandres proposed the idea that hormones regulate the production of red blood cells. After conducting experiments on rabbits subject to bloodletting, Carnot and DeFlandre attributed an increase in red blood cells in rabbit subjects to a hemotropic factor called hemopoietin. Eva Bonsdorff and Eeva Jalavisto continued to study red cell production and later called the hemopoietic substance ‘erythropoietin’. Further studies investigating the existence of EPO by Reissman, and Erslev demonstrated that a certain substance, circulated in the blood, is able to stimulate red blood cell production and increase hematocrit. This substance was finally purified and confirmed as erythropoietin, opening doors to therapeutic uses for EPO in diseases like anemia.[3][4]
Haematologist John Adamson and nephrologist Joseph W. Eschbach looked at various forms of renal failure and the role of the natural hormone EPO in the formation of red blood cells. Studying sheep and other animals in the 1970s, the two scientists helped establish that EPO stimulates the production of red cells in bone marrow and could lead to a treatment for anemia in humans. In 1968, Goldwasser and Kung began work to purify human EPO, and managed to purify milligram quantities of over 95% pure material by 1977.[5] Pure EPO allowed the amino acid sequence to be partially identified and the gene to be isolated.[6] Later an NIH-funded researcher at Columbia University discovered a way to synthesize EPO. Columbia University patented the technique, and licensed it to Amgen. Controversy has ensued over the fairness of the rewards that Amgen reaped from NIH-funded work, and Goldwasser was never financially rewarded for his work.[7]
In the 1980s, Adamson, Joseph W. Eschbach, Joan C. Egrie, Michael R. Downing and Jeffrey K. Browne conducted a clinical trial at the Northwest Kidney Centers for a synthetic form of the hormone, Epogen produced by Amgen. The trial was successful, and the results were published in the New England Journal of Medicine in January 1987.[8]
In 1985, Lin et al. isolated the human erythropoietin gene from a genomic phage library and were able to characterize it for research and production.[9] Their research demonstrated that the gene for erythropoietin encoded the production of EPO in mammalian cells that is biologically active in vitro and in vivo. The industrial production of recombinant human erythropoietin (RhEpo) for treating anemia patients would begin soon after.
In 1989, the U.S. Food and Drug Administration approved the hormone, called Epogen, which remains in use today.
Novel erythropoiesis stimulating protein
More recently, a novel erythropoiesis-stimulating protein (NESP) has been produced.[10] This glycoprotein demonstrates anti-anemic capabilities and has a longer terminal half-life than erythropoietin. NESP offers chronic renal failure patients a lower dose of hormones to maintain normal hemoglobin levels.
Regulation
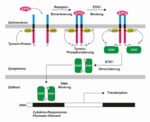
EPO is produced mainly by peritubular capillary lining cells of the renal cortex; which are highly specialized epithelial-like cells. It is synthesized by renal peritubular cells in adults, with a small amount being produced in the liver.[11][12] Regulation is believed to rely on a feed-back mechanism measuring blood oxygenation. Constitutively synthesized transcription factors for EPO, known as hypoxia-inducible factors (HIFs), are hydroxylated and proteosomally digested in the presence of oxygen.[6] It binds to the erythropoietin receptor (EpoR) on the red cell surface and activates a JAK2 cascade. This receptor is also found in a large number of tissues such as bone marrow cells and peripheral/central nerve cells, many of which activate intracellular biological pathways upon binding with Epo.
Primary role in red cell blood line
Erythropoietin has its primary effect on red blood cells by promoting red blood cell survival through protecting these cells from apoptosis. It also cooperates with various growth factors involved in the development of precursor red cells. Specifically, the colony forming unit-erythroid (CFU-E) is completely dependent on erythropoietin. The burst forming unit-erythroid (BFU-E) is also responsive to erythropoietin.
Under hypoxic conditions, the kidney will produce and secrete erythropoietin to increase the production of red blood cells by targeting CFU-E, pro-erythroblast and basophilic erythroblast subsets in the differentiation.
It has a range of actions including vasoconstriction-dependent hypertension, stimulating angiogenesis, and inducing proliferation of smooth muscle fibers. It has also been shown that erythropoietin can increase iron absorption by suppressing the hormone hepcidin.[13]
Uses
Erythropoietin is available as a therapeutic agent produced by recombinant DNA technology in mammalian cell culture. It is used in treating anemia resulting from chronic kidney disease and myelodysplasia, from the treatment of cancer (chemotherapy and radiation). Current research suggests that, aminoacid R103 to E mutation in Erythropoietin makes it Neuroprotective and non-erythropoietic.
Available forms as biomedicine
- Erypro Safe, made by Biocon Ltd.
- Repoitin, made by Serum Institute of India Limited
- Eprex, made by Janssen-Cilag
- NeoRecormon, made by Hoffmann–La Roche
- Vintor, made by Emcure Pharmaceuticals
- Epofit, made by Intas pharma
- Erykine, made by Intas Biopharmaceutica
- Wepox, made by Wockhardt Biotech
- Epogen, made by Amgen
- Espogen, made by LG life sciences.
- ReliPoietin, made by Reliance Life Sciences
- Shanpoietin, made by Shantha Biotechnics Ltd
- Zyrop, made by Cadila Healthcare Ltd.
- Epotrust, made by Panacea Biotec
Anemia due to chronic kidney disease
In patients who require dialysis (have stage 5 chronic kidney disease(CKD)), iron should be given with erythropoietin.[14] Dialysis patients in the US are most often given Epogen; outside of the US other brands of epoetin may be used.
Outside of people on dialysis, erythropoietin is used most commonly to treat anemia in people with chronic kidney disease who are not on dialysis (those in stage 3 or 4 CKD and those living with a kidney transplant). There are two types of erythropoietin for people with anemia due to chronic kidney disease (not on dialysis):
Brands in Epoetin Alpha are:
- Epofit (Intas pharma)
- Epoetin (Procrit (also known as Eprex),
- Darbepoetin (Aranesp)
Brands in Epoetin Beta are:
- NeoRecormon is Epoetin Beta
- MIRCERA is Methoxy Polyethylene Glycol-Epoetin Beta
Anemia due to treatment for cancer
In March 2008, a panel of advisers for the U.S. Food and Drug Administration (FDA) supported keeping ESAs from Amgen and Johnson & Johnson on the market for use in cancer patients. The FDA has focused its concern on study results showing an increased risk of death and tumor growth in chemo patients taking the anti-anemia drugs. According to the FDA, evidence for increased rates of mortality exist in various cancers, including breast, lymphoid, cervical, head and neck, and non-small-cell lung cancer.[15]
Anemia in critically ill patients
There are two types of erythropoietin (and several brands) for people with anemia, due to critical illness. These are:
- Epoetin (Procrit (also known as Eprex) or NeoRecormon)[16]
- Darbepoetin (Aranesp)[17]
- Epoetin delta (Dynepo)
- PDpoetin (an erythropoietin produced in Iran by Pooyesh Darou pharmaceuticals)
- Methoxy polyethylene glycol-epoetin beta (Mircera) by Roche
In a randomized controlled trial,[18] erythropoietin was shown to not change the number of blood transfusions required by critically ill patients. A surprising finding in this study was a small mortality reduction in patients receiving erythropoietin. This result was statistically significant after 29 days but not at 140 days. This mortality difference was most marked in patients admitted to the ICU for trauma. The authors speculate several hypotheses for potential etiologies of this reduced mortality, but, given the known increase in thrombosis and increased benefit in trauma patients as well as marginal nonsignificant benefit (adjusted hazard ratio of 0.9) in surgery patients, it could be speculated that some of the benefit might be secondary to the procoagulant effect of erythropoetin. Regardless, this study suggests further research may be necessary to see which critical care patients, if any, might benefit from administration of erythropoeitin. Any benefit of erythropoetin must be weighed against the 50% increase in thrombosis, which has been demonstrated in numerous trials[citation needed].
Treating anemia in patients undergoing cardiac surgery
In anemic patients undergoing valvular cardiac surgery, with anemia being defined as hemoglobin concentration less than 12 g/dL in women and less than 13 g/dL in men, patients who received recombinant human erythropoietin 16-24 hr before surgery received approximately 1 less unit of blood; of those who were transfused, they received about 1.5 less units of blood, and ten fewer patients needed a transfusion.[19] Furthermore, in the recombinant human erythropoietin group, the lowest postoperative hemoglobin concentration was higher on postoperative days 2, 3 and 4; additionally, reticulocyte count drop was less and erythropoiesis enhancement was greater.[19]
Blood doping
ESAs have a history of use as blood doping agents in endurance sports such as horseracing, cycling, rowing, distance running, race walking, cross country skiing, biathlon, and triathlons.
Though EPO was believed to be widely used in the 1990s in certain sports, there was no way at the time to directly test for it, until in 2000, when a test developed by scientists at the French national anti-doping laboratory (LNDD) and endorsed by the World Anti-Doping Agency (WADA) was introduced to detect pharmaceutical EPO by distinguishing it from the nearly identical natural hormone normally present in an athlete’s urine.
In 2002, at the Winter Olympic Games in Salt Lake City, Don Catlin, MD, the founder and then-director of the UCLA Olympic Analytical Lab, reported finding darbepoetin alfa, a form of erythropoietin, in a test sample for the first time in sports.[20]
In 2010, Floyd Landis admitted to using performance-enhancing drugs, including EPO, throughout the majority of his career as a professional cyclist.[21]
Since 2002, EPO tests performed by U.S. sports authorities have consisted of only a urine or “direct” test. From 2000–2006, EPO tests at the Olympics were conducted on both blood and urine.[22][23]
Neurological diseases
Erythropoietin has been shown to be beneficial in certain neurological diseases like schizophrenia.[24] Research has suggested that EPO improves the survival rate in children suffering from cerebral malaria, caused by the malaria parasite's blocking of blood vessels in the brain.[25][26][27]
Treating patients with chronic pain
Certain analogs of erythropoietin, e.g., ARA290, an 11-amino-acid peptide that mimics the tertiary structure of erythropoietin, can activate the erythropoietin receptor but not erythrocyte precursor cells. In one study, mice and rats underwent either spared nerve injury (lesion of two of the three terminal branches of the sciatic nerve) or a sham operation.[28] Those animals that underwent spared nerve injury were assigned to one of three groups and received intraperitoneal injections and then maintenance, intraperitoneal injections and no maintenance, or sham injection of ARA290. Spared nerve injury caused tactile and cold allodynia (increased sensitivity to non-painful stimuli) and animals that also received ARA290 had long-term relief of tactile and cold allodynia. A decrease in nociceptive and cold threshold was seen in the opposite paw and that response was lesser in animals treated with ARA290. There was less progression of tactile and cold allodynia in rats also treated with maintenance ARA290. There were no ARA290 effects in animals that lacked the EPOR-ßcR complex. If the same is found in humans, this would represent a novel way to treat this debilitating condition.[28]
Adverse effects
Erythropoietin is associated with an increased risk of adverse cardiovascular complications in patients with kidney disease if it is used to increase hemoglobin levels above 13.0 g/dl.[29]
Early treatment with erythropoietin correlated with an increase in the risk of Retinopathy of prematurity in premature infants who had anemia of prematurity, raising concern that the angiogenic actions of erythropoietin may exacerbate retinopathy.[30][31] However, since anemia itself increases the risk of retinopathy, the correlation with erythropoietin treatment may be incidental, and merely reflect that anemia induces retinopathy.
Safety advisories in anemic cancer patients
Amgen sent a "dear doctor" letter in January 2007 that highlighted results from a recent anemia of cancer trial, and warned doctors to consider use in that off-label indication with caution.
Amgen advised the U.S. Food and Drug Administration (FDA) regarding the results of the DAHANCA 10 clinical trial. The DAHANCA 10 data monitoring committee found that 3-year loco-regional cancer control in subjects treated with Aranesp was significantly worse than for those not receiving Aranesp (p=0.01).
In response to these advisories, the FDA released a Public Health Advisory[32] on March 9, 2007, and a clinical alert[33] for doctors on February 16, 2007, about the use of erythropoeisis-stimulating agents (ESAs) such as epogen and darbepoetin. The advisory recommended caution in using these agents in cancer patients receiving chemotherapy or off chemotherapy, and indicated a lack of clinical evidence to support improvements in quality of life or transfusion requirements in these settings.
In addition, on March 9, 2007, drug manufacturers agreed to new black box warnings about the safety of these drugs.
On March 22, 2007, a congressional inquiry into the safety of erythropoeitic growth factors was reported in the news media. Manufacturers were asked to suspend drug rebate programs for physicians and to also suspend marketing the drugs to patients.
Several publications and FDA communications have increased the level of concern related to adverse effects of ESA therapy in selected groups. In a revised Black Box Warning, the FDA notes significant risks associated with ESA use. ESAs should be used only in patients with cancer when treating anemia specifically caused by chemotherapy, and not for other causes of anemia. Further, it states that ESAs should be discontinued once the patient's chemotherapy course has been completed.[34][35][36][37]
Interactions
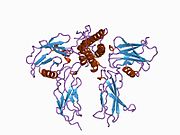
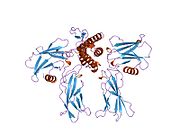
Erythropoietin has been shown to interact with the Erythropoietin receptor as its mechanism of action within the body.[38][39]
Drug interactions with Erythropoietin include: Major interaction:Lenalidomide--risk of thrombosis Moderate interaction:Cyclosporine--risk of high blood pressure may be greater in combination with EPO. EPO may lead to variability in blood levels of cyclosporine. Minor interactions: ACE inhibitors may interfere with hematopoiesis by decreasing the synthesis of endogenous erythropoietin or decreasing bone marrow production of red blood cells. [40]
See also
- Erythropoiesis
- Hemopoietic growth factors
- Amgen, producer of artificial EPO (Brand Names: Epogen and Aranesp)
- Dynepo, trademark name for an erythropoiesis stimulating protein, by TKT
- Blood doping, transfusions and EPO use as doping methods; testing and enforcement
- Jehovah's Witnesses and blood transfusions
Additional images
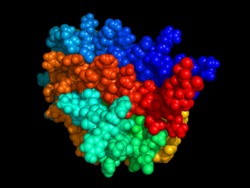
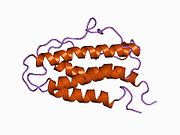
EPO hematopoiesis (German)JAK-STAT signaling pathway (German)EPO structureEPO salesEpo blotting (German)References
- ^ Siren AL et al. (2001). "Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress". Proc Natl Acad Sci USA 98 (7): 4044–4049. doi:10.1073/pnas.051606598. PMC 31176. PMID 11259643. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=31176.
- ^ Haroon ZA, Amin K, Jiang X, Arcasoy MO (September 2003). "A novel role for erythropoietin during fibrin-induced wound-healing response". Am. J. Pathol. 163 (3): 993–1000. PMC 1868246. PMID 12937140. http://ajp.amjpathol.org/cgi/content/abstract/163/3/993.
- ^ Jelkmann W (March 2007). "Erythropoietin after a century of research: younger than ever". European journal of haematology 78 (3): 183–205. doi:10.1111/j.1600-0609.2007.00818.x. PMID 17253966.
- ^ Ahmet Höke (2005). Erythropoietin and the Nervous System. Berlin: Springer. ISBN 0-387-30010-4. OCLC 64571745. http://books.google.com/?id=A76u7g0QnskC.
- ^ Miyake T; Kung, CK; Goldwasser, E (Aug 1997). "Purification of human erythropoietin". J. Biol. Chem. 252 (15): 5558–5564. PMID 18467. http://www.jbc.org/content/252/15/5558.long.
- ^ a b Jelkmann W (March 2007). "Erythropoietin after a century of research: younger than ever". Eur. J. Haematol. 78 (3): 183–205. doi:10.1111/j.1600-0609.2007.00818.x. PMID 17253966.
- ^ Angell, Marcia (2005). The Truth About the Drug Companies : How They Deceive Us and What to Do About It. New York: Random House Trade Paperbacks. p. 60. ISBN 0-375-76094-6.
- ^ Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW (January 1987). "Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial". N. Engl. J. Med. 316 (2): 73–8. doi:10.1056/NEJM198701083160203. PMID 3537801.
- ^ Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z (November 1985). "Cloning and expression of the human erythropoietin gene". Proc. Natl. Acad. Sci. U.S.A. 82 (22): 7580–4. doi:10.1073/pnas.82.22.7580. PMC 391376. PMID 3865178. http://www.pnas.org/content/82/22/7580.abstract.
- ^ Macdougall IC (July 2000). "Novel erythropoiesis stimulating protein". Semin. Nephrol. 20 (4): 375–81. PMID 10928340.
- ^ Jacobson LO, Goldwasser E, Fried W, Plzak L (March 1957). "Role of the kidney in erythropoiesis". Nature 179 (4560): 633–4. doi:10.1038/179633a0. PMID 13418752.
- ^ Fisher JW, Koury S, Ducey T, Mendel S (October 1996). "Erythropoietin production by interstitial cells of hypoxic monkey kidneys". British journal of haematology 95 (1): 27–32. doi:10.1046/j.1365-2141.1996.d01-1864.x. PMID 8857934.
- ^ Ashby DR, Gale DP, Busbridge M, et al. (March 2010). "Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin". Haematologica 95 (3): 505–8. doi:10.3324/haematol.2009.013136. PMC 2833083. PMID 19833632. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2833083.
- ^ Macdougall IC, Tucker B, Thompson J, Tomson CR, Baker LR, Raine AE (1996). "A randomized controlled study of iron supplementation in patients treated with erythropoietin". Kidney Int. 50 (5): 1694–9. doi:10.1038/ki.1996.487. PMID 8914038.
- ^ Smith A (2008-03-13). "FDA panel gives surprise OK to Amgen and J&J: FDA panelists support keeping Amgen, J&J drugs on market - Mar. 13, 2008". CNNMoney.com. http://money.cnn.com/2008/03/13/news/companies/amgen/?postversion=2008031317. Retrieved 2009-03-31.
- ^ "Procrit (Epoetin alfa)". Ortho Biotech Products. http://www.procrit.com/procrit/. Retrieved 2009-04-29.[dead link]
- ^ "Aranesp(darbepoetin alfa)". Amgen.com. http://www.aranesp.com/. Retrieved 2009-04-29.
- ^ Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, Corwin MJ (September 2007). "Efficacy and safety of epoetin alfa in critically ill patients". The New England Journal of Medicine 357 (10): 965–76. doi:10.1056/NEJMoa071533. PMID 17804841.
- ^ a b Yoo YC, Shim JK, Kim JC, Jo YY, Lee JH, Kwak YL (November 2011). "Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery". Anesthesiology 115 (5): 929–37. doi:10.1097/ALN.0b013e318232004b. PMID 22027622. Lay summary – Anesthesiology's blog for education & more.
- ^ Steeg JL (2007-02-28). "Catlin has made a career out of busting juicers - USATODAY.com". USA TODAY. http://www.usatoday.com/sports/olympics/2007-02-28-catlin-drug-lab_N.htm. Retrieved 2009-03-31.
- ^ "Landis admits to illegal drug use". BBC News. 2010-05-20. http://news.bbc.co.uk/sport2/hi/other_sports/cycling/8694452.stm.
- ^ Lasne F, Martin L, Crepin N, de Ceaurriz J (December 2002). "Detection of isoelectric profiles of erythropoietin in urine: differentiation of natural and administered recombinant hormones". Anal. Biochem. 311 (2): 119–26. doi:10.1016/S0003-2697(02)00407-4. PMID 12470670.
- ^ Kohler M, Ayotte C, Desharnais P, Flenker U, Lüdke S, Thevis M, Völker-Schänzer E, Schänzer W (January 2008). "Discrimination of recombinant and endogenous urinary erythropoietin by calculating relative mobility values from SDS gels". Int J Sports Med 29 (1): 1–6. doi:10.1055/s-2007-989369. PMID 18050057.
- ^ Ehrenreich H, Degner D, Meller J, et al. (January 2004). "Erythropoietin: a candidate compound for neuroprotection in schizophrenia" (PDF). Molecular psychiatry 9 (1): 42–54. doi:10.1038/sj.mp.4001442. PMID 14581931. http://physiologie.univ-lyon1.fr/enseignement/coursLB/Ehrenreich2004.pdf.
- ^ Casals-Pascual C, Idro R, Picot S, Roberts DJ, Newton CR (2009). "Can erythropoietin be used to prevent brain damage in cerebral malaria?". Trends Parasitol 25 (1): 30–6. doi:10.1016/j.pt.2008.10.002. PMID 19008152. http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19008152.
- ^ Core A, Hempel C, Kurtzhals JA, Penkowa M (2011). "Plasmodium berghei ANKA: erythropoietin activates neural stem cells in an experimental cerebral malaria model.". Exp Parasitol 127 (2): 500–5. doi:10.1016/j.exppara.2010.09.010. PMID 21044627. http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21044627.
- ^ "Kidney drug could save children from malaria brain damage". The Guardian. http://www.guardian.co.uk/science/2008/feb/17/medicalresearch.health1.
- ^ a b Swartjes M, Morariu A, Niesters M, Brines M, Cerami A, Aarts L, Dahan A (November 2011). "ARA290, a Peptide Derived from the Tertiary Structure of Erythropoietin, Produces Long-term Relief of Neuropathic Pain: An Experimental Study in Rats and β-Common Receptor Knockout Mice". Anesthesiology 115 (5): 1084–92. doi:10.1097/ALN.0b013e31822fcefd. PMID 21873879. Lay summary – Anesthesiology's blog for education & more.
- ^ Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A (2006). "Normalization of hemoglobin level in patients with chronic kidney disease and anemia". N. Engl. J. Med. 355 (20): 2071–84. doi:10.1056/NEJMoa062276. PMID 17108342.
- ^ Ohlsson A, Aher SM (2006). "Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants". Cochrane Database Syst Rev 3: CD004863. doi:10.1002/14651858.CD004863.pub2. PMID 16856062.
- ^ Aher SM, Ohlsson A (2006). "Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants". Cochrane Database Syst Rev 3: CD004865. doi:10.1002/14651858.CD004865.pub2. PMID 16856063.
- ^ "FDA Public Health Advisory: Erythropoiesis-Stimulating Agents (ESAs): Epoetin alfa (marketed as Procrit, Epogen), Darbepoetin alfa (marketed as Aranesp)". Archived from the original on 2007-05-28. http://web.archive.org/web/20070528103823/http://www.fda.gov/cder/drug/advisory/RHE2007.htm. Retrieved 2007-06-05.
- ^ "Information for Healthcare Professionals: Erythropoiesis Stimulating Agents (ESA)". Archived from the original on 2007-05-15. http://web.archive.org/web/20070515034509/http://www.fda.gov/cder/drug/InfoSheets/HCP/RHE2007HCP.htm. Retrieved 2007-06-05.
- ^ "Erythropoiesis Stimulating Agents: Aranesp (darbepoetin alfa), Epogen (epoetin alfa), and Procrit (epoetin alfa)". MedWatch - 2007 Safety Information Alerts. U.S. Food and Drug Administration. 2008-01-03. http://www.fda.gov/medwatch/safety/2007/safety07.htm#ESA2. Retrieved 2009-04-09.
- ^ "Procrit (Epoetin alfa) for injection". U.S. Food and Drug Administration. 2007-08-11. http://www.fda.gov/cder/foi/label/2007/103234s5158lbl.pdf. Retrieved 2009-04-09.[dead link]
- ^ "Aranesp (darbepoetin alfa) for Injection". U.S. Food and Drug Administration. 2007-11-08. http://www.fda.gov/cder/foi/label/2007/103951s5164lbl.pdf. Retrieved 2009-04-09.[dead link]
- ^ "Information on Erythropoiesis Stimulating Agents (ESA) (marketed as Procrit, Epogen, and Aranesp)". U.S. Food and Drug Administration. 2009-01-26. http://www.fda.gov/cder/drug/infopage/RHE/default.htm. Retrieved 2009-04-09.
- ^ Middleton, S A; Barbone F P, Johnson D L, Thurmond R L, You Y, McMahon F J, Jin R, Livnah O, Tullai J, Farrell F X, Goldsmith M A, Wilson I A, Jolliffe L K (May. 1999). "Shared and unique determinants of the erythropoietin (EPO) receptor are important for binding EPO and EPO mimetic peptide". J. Biol. Chem. (UNITED STATES) 274 (20): 14163–9. doi:10.1074/jbc.274.20.14163. ISSN 0021-9258. PMID 10318834.
- ^ Livnah, O; Johnson D L, Stura E A, Farrell F X, Barbone F P, You Y, Liu K D, Goldsmith M A, He W, Krause C D, Pestka S, Jolliffe L K, Wilson I A (Nov. 1998). "An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation". Nat. Struct. Biol. (UNITED STATES) 5 (11): 993–1004. doi:10.1038/2965. ISSN 1072-8368. PMID 9808045.
- ^ Drug Interactions of Erythropoietin Alfa at Drugs.com
Further reading
- Takeuchi M, Kobata A (1992). "Structures and functional roles of the sugar chains of human erythropoietins.". Glycobiology 1 (4): 337–46. doi:10.1093/glycob/1.4.337. PMID 1820196.
- Semba RD, Juul SE (2002). "Erythropoietin in human milk: physiology and role in infant health.". Journal of human lactation : official journal of International Lactation Consultant Association 18 (3): 252–61. PMID 12192960.
- Ratcliffe PJ (2003). "From erythropoietin to oxygen: hypoxia-inducible factor hydroxylases and the hypoxia signal pathway.". Blood Purif. 20 (5): 445–50. doi:10.1159/000065201. PMID 12207089.
- Westenfelder C (2003). "Unexpected renal actions of erythropoietin.". Exp. Nephrol. 10 (5-6): 294–8. doi:10.1159/000065304. PMID 12381912.
- Becerra SP, Amaral J (2002). "Erythropoietin--an endogenous retinal survival factor.". N. Engl. J. Med. 347 (24): 1968–70. doi:10.1056/NEJMcibr022629. PMID 12477950.
- Genc S, Koroglu TF, Genc K (2004). "Erythropoietin and the nervous system.". Brain Res. 1000 (1-2): 19–31. doi:10.1016/j.brainres.2003.12.037. PMID 15053948.
- Fandrey J (2004). "Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression.". Am. J. Physiol. Regul. Integr. Comp. Physiol. 286 (6): R977–88. doi:10.1152/ajpregu.00577.2003. PMID 15142852.
- Juul S (2004). "Recombinant erythropoietin as a neuroprotective treatment: in vitro and in vivo models.". Clinics in perinatology 31 (1): 129–42. doi:10.1016/j.clp.2004.03.004. PMID 15183662.
- Buemi M, Caccamo C, Nostro L, et al. (2005). "Brain and cancer: the protective role of erythropoietin.". Med Res Rev 25 (2): 245–59. doi:10.1002/med.20012. PMID 15389732.
- Sytkowski AJ (2007). "Does erythropoietin have a dark side? Epo signaling and cancer cells.". Sci. STKE 2007 (395): e38. doi:10.1126/stke.3952007pe38. PMID 17636183.
External links
PDB gallery Endocrine system: hormones (Peptide hormones · Steroid hormones) Endocrine
glandsTestis: testosterone · AMH · inhibin
Ovary: estradiol · progesterone · activin and inhibin · relaxin (pregnancy)
Placenta: hCG · HPL · estrogen · progesteroneIslet-Acinar
AxisNon-end.
glandsThymus: Thymosin (Thymosin α1, Thymosin beta) · Thymopoietin · Thymulin
Digestive system: Stomach: gastrin · ghrelin · Duodenum: CCK · GIP · secretin · motilin · VIP · Ileum: enteroglucagon · peptide YY · Liver/other: Insulin-like growth factor (IGF-1, IGF-2)
Adipose tissue: leptin · adiponectin · resistin
Kidney: JGA (renin) · peritubular cells (EPO) · calcitriol · prostaglandin
Heart: Natriuretic peptide (ANP, BNP)Urinary system, physiology: renal physiology and acid-base physiology Filtration Hormones affecting filtration Secretion/clearance Reabsorption Endocrine Assessing Renal function/
Measures of dialysisGlomerular filtration rate · Creatinine clearance · Renal clearance ratio · Urea reduction ratio · Kt/V · Standardized Kt/V · Hemodialysis product · PAH clearance (Effective renal plasma flow · Extraction ratio)Acid-base physiology Fluid balance · Darrow Yannet diagram
Body water: Intracellular fluid/Cytosol · Extracellular fluid · (Interstitial fluid · Plasma · Transcellular fluid)
Base excess · Davenport diagram · Anion gap · Arterial blood gas · Winter's formulaBuffering/compensation Other CFU-GEMM CFU-GM CFU-E ErythropoietinCFU-Meg Categories:- Human proteins
- Growth factors
- Hormones of the kidneys
- Amgen
- Drugs in sport
- Antianemic preparations
- Erythropoiesis-stimulating agents
Wikimedia Foundation. 2010.