- Clearance (medicine)
-
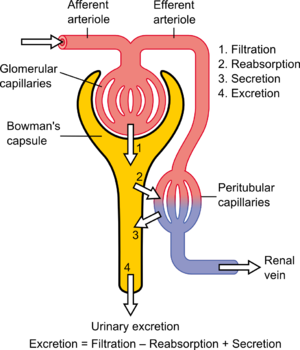
In medicine, the clearance is a measurement of the renal excretion ability. Although clearance may also involve other organs than the kidney, it is almost synonymous with renal clearance or renal plasma clearance. Each substance has a specific clearance that depends on its filtration characteristics. Clearance is a function of glomerular filtration, secretion from the peritubular capillaries to the nephron, and reabsorption from the nephron back to the peritubular capillaries. Clearance is constant in first-order kinetics because a constant fraction of the drug is eliminated per unit time, but it is variable in zero-order kinetics, because a constant amount of drug is eliminated per unit time[1][2].
Contents
Definition
When referring to the function of the kidney, clearance is considered to be the amount of liquid filtered out of the blood that gets processed by the kidneys or the amount of blood cleaned per time because it has the units of a volumetric flow rate [ volume / time ]. However, it does not refer to a real value; "[t]he kidney does not completely remove a substance from the total renal plasma flow."[3] From a mass transfer perspective[4] and physiologically, volumetric blood flow (to the dialysis machine and/or kidney) is only one of several factors that determine blood concentration and removal of a substance from the body. Other factors include the mass transfer coefficient, dialysate flow and dialysate recirculation flow for hemodialysis, and the glomerular filtration rate and the tubular reabsorption rate, for the kidney. A physiologic interpretation of clearance (at steady-state) is that clearance is a ratio of the mass generation and blood (or plasma) concentration.
Its definition follows from the differential equation that describes exponential decay and is used to model kidney function and hemodialysis machine function:

Where:
 is the mass generation rate of the substance - assumed to be a constant, i.e. not a function of time (equal to zero for foreign substances/drugs) [mmol/min] or [mol/s]
is the mass generation rate of the substance - assumed to be a constant, i.e. not a function of time (equal to zero for foreign substances/drugs) [mmol/min] or [mol/s]- t is dialysis time or time since injection of the substance/drug [min] or [s]
- V is the volume of distribution or total body water [L] or [m³]
- K is the clearance [mL/min] or [m³/s]
- C is the concentration [mmol/L] or [mol/m³] (in the USA often [mg/mL])
From the above definitions it follows that
 is the first derivative of concentration with respect to time, i.e. the change in concentration with time.
is the first derivative of concentration with respect to time, i.e. the change in concentration with time.It is derived from a mass balance.
Clearance of a substance is sometimes expressed as the inverse of the time constant that describes its removal rate from the body divided by its volume of distribution (or total body water).
In steady-state, it is defined as the mass generation rate of a substance (which equals the mass removal rate) divided by its concentration in the blood.
Effect of plasma protein binding
For substances that exhibit substantial plasma protein binding, clearance is generally defined as the total concentration (free + protein-bound) and not the free concentration.[5]
Most plasma substances have primarily their free concentrations regulated, which thus remains the same, so extensive protein binding increases total plasma concentration (free + protein-bound). This gives a decreased clearance than what would have been the case with no protein binding.[5] However, the mass removal rate is the same[5], because it depends only on concentration of free substance, and is independent on plasma protein binding, even with the fact that plasma proteins increase in concentration in the distal renal glomerulus as plasma is filtered into Bowman's capsule, because the relative increases in concentrations of substance-protein and non-occupied protein are equal and therefore give no net binding or dissociation of substances from plasma proteins, thus giving a constant plasma concentration of free substance throughout the glomerulus, which also would have been the case without any plasma protein binding.
In other sites than the kidneys, however, where clearance is made by membrane transport proteins rather than filtration, extensive plasma protein binding may increase clearance by keeping concentration of free substance fairly constant throughout the capillary bed, inhibiting a decrease in clearance caused by decreased concentration of free substance through the capillary.
Derivation of equation
Equation 1 is derived from a mass balance:
where:
- Δt is a period of time
- Δmbody the change in mass of the toxin in the body during Δt
 is the toxin intake rate
is the toxin intake rate is the toxin removal rate
is the toxin removal rate is the toxin generation rate
is the toxin generation rate
In words, the above equation states:
- The change in the mass of a toxin within the body (Δm) during some time Δt is equal to the toxin intake plus the toxin generation minus the toxin removal.
Since
and
Equation A1 can be rewritten as:
If one lumps the in and gen. terms together, i.e.
 and divides by Δt the result is a difference equation:
and divides by Δt the result is a difference equation:If one applies the limit
 one obtains a differential equation:
one obtains a differential equation:Using the Product Rule this can be rewritten as:
If one assumes that the volume change is not significant, i.e.
 , the result is Equation 1:
, the result is Equation 1:Solution to the differential equation
The general solution of the above differential equation (1) is:
Where:
- Co is the concentration at the beginning of dialysis or the initial concentration of the substance/drug (after it has distributed) [mmol/L] or [mol/m³]
- e is the base of the natural logarithm
Steady-state solution
The solution to the above differential equation (9) at time infinity (steady state) is:

The above equation (10a) can be rewritten as:

The above equation (10b) makes clear the relationship between mass removal and clearance. It states that (with a constant mass generation) the concentration and clearance vary inversely with one another. If applied to creatinine (i.e. creatinine clearance), it follows from the equation that if the serum creatinine doubles the clearance halves and that if the serum creatinine quadruples the clearance is quartered.
Measurement of renal clearance
Renal clearance can be measured with a timed collection of urine and an analysis of its composition with the aid of the following equation (which follows directly from the derivation of (10b)):

Where:
- K is the clearance [mL/min]
- CU is the urine concentration [mmol/L] (in the USA often [mg/mL])
- Q is the urine flow (volume/time) [mL/min] (often [mL/24 hours])
- CB is the plasma concentration [mmol/L] (in the USA often [mg/mL])
When the substance "C" is creatinine, an endogenous chemical that is excreted only by filtration, the calculated clearance is equivalent to the glomerular filtration rate. Inulin clearance is also used to estimate glomerular filtration rate.
Note - the above equation (11) is valid only for the steady-state condition. If the substance being cleared is not at a constant plasma concentration (i.e. not at steady-state) K must be obtained from the (full) solution of the differential equation (9).
See also
- Table of medication secreted in kidney
- Sieving coefficient
- Creatinine clearance
- Kt/V
- Pharmacokinetics
- Renal clearance ratio
- Standardized Kt/V
- Urea reduction ratio
References
- ^ http://www.pharmacology2000.com/General/Pharmacokinetics/kinobj1.htm
- ^ Kaplan Step1 Pharmacology 2010, page 14
- ^ Seldin DW (2004). "The development of the clearance concept". J. Nephrol. 17 (1): 166–71. PMID 15151274. Available at: http://www.sin-italy.org/jnonline/Vol17n1/166.html. Accessed on: Sept 2, 2007.
- ^ Babb AL, Popovich RP, Christopher TG, Scribner BH (1971). "The genesis of the square meter-hour hypothesis". Transactions - American Society for Artificial Internal Organs 17: 81–91. PMID 5158139.
- ^ a b c Basic clinical pharmacokinetics, Page 32: Plasma protein binding By Michael E. Winter Edition: 4, illustrated Published by Lippincott Williams & Wilkins, 2003 ISBN 0781741475, 9780781741477 511 pages
- ^ Gotch FA (1998). "The current place of urea kinetic modelling with respect to different dialysis modalities". Nephrol. Dial. Transplant. 13 Suppl 6 (90006): 10–4. doi:10.1093/ndt/13.suppl_6.10. PMID 9719197. Full Text
- ^ Gotch FA, Sargent JA, Keen ML (2000). "Whither goest Kt/V?". Kidney Int. Suppl. 76: S3–18. doi:10.1046/j.1523-1755.2000.07602.x. PMID 10936795.
Urinary system, physiology: renal physiology and acid-base physiology Filtration Hormones affecting filtration Secretion/clearance Reabsorption Endocrine Assessing Renal function/
Measures of dialysisGlomerular filtration rate · Creatinine clearance · Renal clearance ratio · Urea reduction ratio · Kt/V · Standardized Kt/V · Hemodialysis product · PAH clearance (Effective renal plasma flow · Extraction ratio)Acid-base physiology Fluid balance · Darrow Yannet diagram
Body water: Intracellular fluid/Cytosol · Extracellular fluid · (Interstitial fluid · Plasma · Transcellular fluid)
Base excess · Davenport diagram · Anion gap · Arterial blood gas · Winter's formulaBuffering/compensation Other Medication > Pharmacology Pharmacokinetics Pharmacodynamics Agonism and antagonism Agonist: Inverse agonist • Irreversible agonist • Partial agonist • Superagonist • Physiological agonist
Antagonist: Competitive antagonist • Irreversible antagonist • Physiological antagonist
Other: Binding • Affinity • Binding selectivity • Functional selectivityOther Related fields/subfields Categories:
Wikimedia Foundation. 2010.