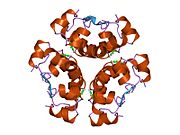
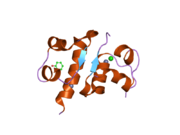- Insulin
-
This article is about the insulin protein. For uses of insulin in treating diabetes, see insulin therapy.
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle.
Insulin stops the use of fat as an energy source by inhibiting the release of glucagon. With the exception of the metabolic disorder diabetes mellitus and Metabolic syndrome, insulin is provided within the body in a constant proportion to remove excess glucose from the blood, which otherwise would be toxic. When blood glucose levels fall below a certain level, the body begins to use stored sugar as an energy source through glycogenolysis, which breaks down the glycogen stored in the liver and muscles into glucose which can then be utilized as an energy source. As its level is a central metabolic control mechanism, its status is also used as a control signal to other body systems (such as amino acid uptake by body cells). In addition, it has several other anabolic effects throughout the body.
When control of insulin levels fails, diabetes mellitus will result. As a consequence, insulin is used medically to treat some forms of diabetes mellitus. Patients with type 1 diabetes depend on external insulin (most commonly injected subcutaneously) for their survival because the hormone is no longer produced internally. Patients with type 2 diabetes are often insulin resistant and, because of such resistance, may suffer from a "relative" insulin deficiency. Some patients with type 2 diabetes may eventually require insulin if other medications fail to control blood glucose levels adequately. Over 40% of those with Type 2 diabetes require insulin as part of their diabetes management plan.
Insulin also influences other body functions, such as vascular compliance and cognition. Once insulin enters the human brain, it enhances learning and memory and benefits verbal memory in particular.[2] Enhancing brain insulin signaling by means of intranasal insulin administration also enhances the acute thermoregulatory and glucoregulatory response to food intake, suggesting that central nervous insulin contributes to the control of whole-body energy homeostasis in humans.[3]
Human insulin is a peptide hormone composed of 51 amino acids and has a molecular weight of 5808 Da. It is produced in the islets of Langerhans in the pancreas. The name comes from the Latin insula for "island". Insulin's structure varies slightly between species of animals. Insulin from animal sources differs somewhat in "strength" (in carbohydrate metabolism control effects) in humans because of those variations. Porcine insulin is especially close to the human version.
Contents
Gene
The proinsulin precursor of insulin is encoded by the INS gene.[4][5]
Alleles
A variety of mutant alleles with changes in the coding region have been identified. A read-through gene, INS-IGF2, overlaps with this gene at the 5' region and with the IGF2 gene at the 3' region.[4]
Regulation
Several regulatory sequences in the promoter region of the human insulin gene bind to transcription factors. In general, the A-boxes bind to Pdx1 factors, E-boxes bind to NeuroD, C-boxes bind to MafA, and cAMP response elements to CREB. There are also silencers that inhibit transcription.
Regulatory sequences and their transcription factors for the insulin gene.[6] Regulatory sequence binding transcription factors ILPR Par1 A5 Pdx1 negative regulatory element (NRE)[7] glucocorticoid receptor, Oct1 Z (overlapping NRE and C2) ISF C2 Pax4, MafA(?) E2 USF1/USF2 A3 Pdx1 CREB RE - CREB RE CREB, CREM A2 - CAAT enhancer binding (CEB) (partly overlapping A2 and C1) - C1 - E1 E2A, NeuroD1, HEB A1 Pdx1 G1 - Protein structure
See also: Insulin/IGF/Relaxin familyWithin vertebrates, the amino acid sequence of insulin is extremely well-preserved. Bovine insulin differs from human in only three amino acid residues, and porcine insulin in one. Even insulin from some species of fish is similar enough to human to be clinically effective in humans. Insulin in some invertebrates is quite similar in sequence to human insulin, and has similar physiological effects. The strong homology seen in the insulin sequence of diverse species suggests that it has been conserved across much of animal evolutionary history. The C-peptide of proinsulin (discussed later), however, differs much more among species; it is also a hormone, but a secondary one.
The primary structure of bovine insulin was first determined by Frederick Sanger in 1951.[8] After that, this polypeptide was synthesized independently by several groups.[9][10][11]
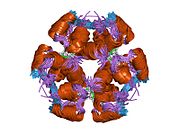
Insulin is produced and stored in the body as a hexamer (a unit of six insulin molecules), while the active form is the monomer. The hexamer is an inactive form with long-term stability, which serves as a way to keep the highly reactive insulin protected, yet readily available. The hexamer-monomer conversion is one of the central aspects of insulin formulations for injection. The hexamer is far more stable than the monomer, which is desirable for practical reasons; however, the monomer is a much faster-reacting drug because diffusion rate is inversely related to particle size. A fast-reacting drug means insulin injections do not have to precede mealtimes by hours, which in turn gives diabetics more flexibility in their daily schedules.[12] Insulin can aggregate and form fibrillar interdigitated beta-sheets. This can cause injection amyloidosis, and prevents the storage of insulin for long periods.[13]
Synthesis, physiological effects, and degradation
Synthesis
Insulin is produced in the pancreas and released when any of several stimuli are detected. These stimuli include ingested protein and glucose in the blood produced from digested food. Carbohydrates can be polymers of simple sugars or the simple sugars themselves. If the carbohydrates include glucose, then that glucose will be absorbed into the bloodstream and blood glucose level will begin to rise. In target cells, insulin initiates a signal transduction, which has the effect of increasing glucose uptake and storage. Finally, insulin is degraded, terminating the response.
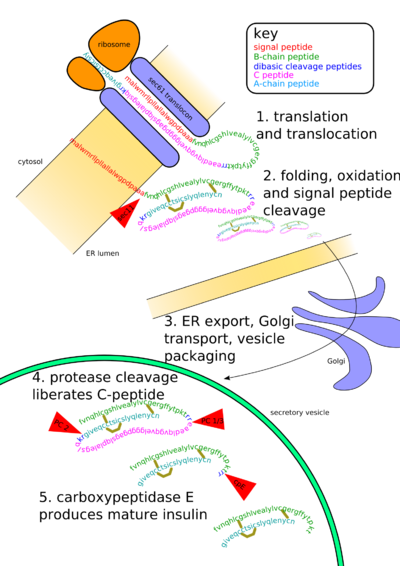 Insulin undergoes extensive posttranslational modification along the production pathway. Production and secretion are largely independent; prepared insulin is stored awaiting secretion. Both C-peptide and mature insulin are biologically active. Cell components and proteins in this image are not to scale.
Insulin undergoes extensive posttranslational modification along the production pathway. Production and secretion are largely independent; prepared insulin is stored awaiting secretion. Both C-peptide and mature insulin are biologically active. Cell components and proteins in this image are not to scale.
In mammals, insulin is synthesized in the pancreas within the β-cells of the islets of Langerhans. One million to three million islets of Langerhans (pancreatic islets) form the endocrine part of the pancreas, which is primarily an exocrine gland. The endocrine portion accounts for only 2% of the total mass of the pancreas. Within the islets of Langerhans, beta cells constitute 60–80% of all the cells.
In β-cells, insulin is synthesized from the proinsulin precursor molecule by the action of proteolytic enzymes, known as prohormone convertases (PC1 and PC2), as well as the exoprotease carboxypeptidase E.[14] These modifications of proinsulin remove the center portion of the molecule (i.e., C-peptide), from the C- and N- terminal ends of proinsulin. The remaining polypeptides (51 amino acids in total), the B- and A- chains, are bound together by disulfide bonds. However, the primary sequence of proinsulin goes in the order "B-C-A", since B and A chains were identified on the basis of mass, and the C-peptide was discovered after the others.
The endogenous production of insulin is regulated in several steps along the synthesis pathway:
- At transcription from the insulin gene
- In mRNA stability
- At the mRNA translation
- In the posttranslational modifications
Insulin and its related proteins have been shown to be produced inside the brain, and reduced levels of these proteins are linked to Alzheimer's disease.[15][16][17]
Release
See also: Blood glucose regulationBeta cells in the islets of Langerhans release insulin in two phases. The first phase release is rapidly triggered in response to increased blood glucose levels. The second phase is a sustained, slow release of newly formed vesicles triggered independently of sugar. The description of first phase release is as follows:
- Glucose enters the β-cells through the glucose transporter GLUT2
- Glucose goes into glycolysis and the respiratory cycle, where multiple high-energy ATP molecules are produced by oxidation
- Dependent on the ATP:ADP ratio, and hence blood glucose levels, the ATP-dependent potassium channels (K+) close and the cell membrane depolarizes
- On depolarization, voltage-controlled calcium channels (Ca2+) open and calcium flows into the cells
- An increased calcium level causes activation of phospholipase C, which cleaves the membrane phospholipid phosphatidyl inositol 4,5-bisphosphate into inositol 1,4,5-triphosphate and diacylglycerol.
- Inositol 1,4,5-triphosphate (IP3) binds to receptor proteins in the membrane of endoplasmic reticulum (ER). This allows the release of Ca2+ from the ER via IP3-gated channels, and further raises the cell concentration of calcium.
- Significantly increased amounts of calcium in the cells causes release of previously synthesized insulin, which has been stored in secretory vesicles
This is the main mechanism for release of insulin. Also, in general, some release takes place with food intake, not just glucose or carbohydrate intake, and the β-cells are also somewhat influenced by the autonomic nervous system. The signaling mechanisms controlling these linkages are not fully understood.
Other substances known to stimulate insulin release include amino acids from ingested proteins, acetylcholine released from vagus nerve endings (parasympathetic nervous system), gastrointestinal hormones released by enteroendocrine cells of intestinal mucosa and glucose-dependent insulinotropic peptide (GIP). Three amino acids (alanine, glycine, and arginine) act similarly to glucose by altering the β-cell's membrane potential. Acetylcholine triggers insulin release through phospholipase C, whereas the last acts through the mechanism of adenylate cyclase.
The sympathetic nervous system (via α2-adrenergic stimulation as demonstrated by the agonists clonidine or methyldopa) inhibit the release of insulin. However, it is worth noting that circulating adrenaline will activate β2-receptors on the β-cells in the pancreatic islets to promote insulin release.[citation needed] This is important, since muscle cannot benefit from the raised blood sugar resulting from adrenergic stimulation (increased gluconeogenesis and glycogenolysis from the low blood insulin: glucagon state) unless insulin is present to allow for GLUT-4 translocation in the tissue. Therefore, beginning with direct innervation, norepinephrine inhibits insulin release via α2-receptors, then subsequently, circulating adrenaline from the adrenal medulla will stimulate β2-receptors, thereby promoting insulin release.
When the glucose level comes down to the usual physiologic value, insulin release from the β-cells slows or stops. If blood glucose levels drop lower than this, especially to dangerously low levels, release of hyperglycemic hormones (most prominently glucagon from islet of Langerhans alpha cells) forces release of glucose into the blood from cellular stores, primarily liver cell stores of glycogen. By increasing blood glucose, the hyperglycemic hormones prevent or correct life-threatening hypoglycemia. Release of insulin is strongly inhibited by the stress hormone norepinephrine (noradrenaline), which leads to increased blood glucose levels during stress.
Evidence of impaired first-phase insulin release can be seen in the glucose tolerance test, demonstrated by a substantially elevated blood glucose level at 30 minutes, a marked drop by 60 minutes, and a steady climb back to baseline levels over the following hourly time points.
Oscillations
Even during digestion, in general, one or two hours following a meal, insulin release from the pancreas is not continuous, but oscillates with a period of 3–6 minutes, changing from generating a blood insulin concentration more than about 800 pmol/l to less than 100 pmol/l.[18] This is thought to avoid downregulation of insulin receptors in target cells, and to assist the liver in extracting insulin from the blood.[18] This oscillation is important to consider when administering insulin-stimulating medication, since it is the oscillating blood concentration of insulin release, which should, ideally, be achieved, not a constant high concentration.[18] This may be achieved by delivering insulin rhythmically to the portal vein or by islet cell transplantation to the liver.[18] Future insulin pumps hope to address this characteristic. (See also Pulsatile Insulin.)
Blood content
 The idealized diagram shows the fluctuation of blood sugar (red) and the sugar-lowering hormone insulin (blue) in humans during the course of a day containing three meals. In addition, the effect of a sugar-rich versus a starch-rich meal is highlighted.
The idealized diagram shows the fluctuation of blood sugar (red) and the sugar-lowering hormone insulin (blue) in humans during the course of a day containing three meals. In addition, the effect of a sugar-rich versus a starch-rich meal is highlighted.
The blood content of insulin can be measured in international units, such as µIU/mL or in molar concentration, such as pmol/L, where 1 µIU/mL equals 6.945 pmol/l.[19] A typical blood level between meals is 8–11 μIU/ml (57–79 pmol/l).[20]
Signal transduction
Special transporter proteins in cell membranes allow glucose from the blood to enter a cell. These transporters are, indirectly, under blood insulin's control in certain body cell types (e.g., muscle cells). Low levels of circulating insulin, or its absence, will prevent glucose from entering those cells (e.g., in type 1 diabetes). More commonly, however, there is a decrease in the sensitivity of cells to insulin (e.g., the reduced insulin sensitivity characteristic of type 2 diabetes), resulting in decreased glucose absorption. In either case, there is 'cell starvation' and weight loss, sometimes extreme. In a few cases, there is a defect in the release of insulin from the pancreas. Either way, the effect is the same: elevated blood glucose levels.
Activation of insulin receptors leads to internal cellular mechanisms that directly affect glucose uptake by regulating the number and operation of protein molecules in the cell membrane that transport glucose into the cell. The genes that specify the proteins that make up the insulin receptor in cell membranes have been identified, and the structures of the interior, transmembrane section, and the extra-membrane section of receptor have been solved.
Two types of tissues are most strongly influenced by insulin, as far as the stimulation of glucose uptake is concerned: muscle cells (myocytes) and fat cells (adipocytes). The former are important because of their central role in movement, breathing, circulation, etc., and the latter because they accumulate excess food energy against future needs. Together, they account for about two-thirds of all cells in a typical human body.
Insulin binds to the extracellular portion of the alpha subunits of the insulin receptor. This, in turn, causes a conformational change in the insulin receptor that activates the kinase domain residing on the intracellular portion of the beta subunits. The activated kinase domain autophosphorylates tyrosine residues on the C-terminus of the receptor as well as tyrosine residues in the IRS-1 protein.
- phosphorylated IRS-1, in turn, binds to and activates phosphoinositol 3 kinase (PI3K)
- PI3K catalyzes the reaction PIP2 + ATP → PIP3
- PIP3 activates protein kinase B (PKB)
- PKB phosphorylates glycogen synthase kinase (GSK) and thereby inactivates GSK[21]
- GSK can no longer phosphorylate glycogen synthase (GS)
- unphosphorylated GS makes more glycogen
- PKB also facilitates vesicle fusion, resulting in an increase in GLUT4 transporters in the plasma membrane[22]
Low-frequency internal motion
See also: Low-frequency collective motion in proteins and DNAAccording to the study of Raman spectra, a low-frequency wave number of 22 cm−1 has been observed for insulin molecules.[23] Subsequently, it was identified as the accordion-like vibration of the helix (B9-B19) in the B-chain of insulin.[24][25]
Physiological effects
 Effect of insulin on glucose uptake and metabolism. Insulin binds to its receptor (1), which starts many protein activation cascades (2). These include translocation of Glut-4 transporter to the plasma membrane and influx of glucose (3), glycogen synthesis (4), glycolysis (5) and fatty acid synthesis (6).
Effect of insulin on glucose uptake and metabolism. Insulin binds to its receptor (1), which starts many protein activation cascades (2). These include translocation of Glut-4 transporter to the plasma membrane and influx of glucose (3), glycogen synthesis (4), glycolysis (5) and fatty acid synthesis (6).
The actions of insulin on the global human metabolism level include:
- Control of cellular intake of certain substances, most prominently glucose in muscle and adipose tissue (about two-thirds of body cells)
- Increase of DNA replication and protein synthesis via control of amino acid uptake
- Modification of the activity of numerous enzymes
The actions of insulin (indirect and direct) on cells include:
- Increased glycogen synthesis – insulin forces storage of glucose in liver (and muscle) cells in the form of glycogen; lowered levels of insulin cause liver cells to convert glycogen to glucose and excrete it into the blood. This is the clinical action of insulin, which is directly useful in reducing high blood glucose levels as in diabetes.
- Increased lipid synthesis – insulin forces fat cells to take in blood lipids, which are converted to triglycerides; lack of insulin causes the reverse.
- Increased esterification of fatty acids – forces adipose tissue to make fats (i.e., triglycerides) from fatty acid esters; lack of insulin causes the reverse.
- Decreased proteolysis – decreasing the breakdown of protein
- Decreased lipolysis – forces reduction in conversion of fat cell lipid stores into blood fatty acids; lack of insulin causes the reverse.
- Decreased gluconeogenesis – decreases production of glucose from nonsugar substrates, primarily in the liver (the vast majority of endogenous insulin arriving at the liver never leaves the liver); lack of insulin causes glucose production from assorted substrates in the liver and elsewhere.
- Decreased autophagy - decreased level of degradation of damaged organelles. Postprandial levels inhibit autophagy completely.[26]
- Increased amino acid uptake – forces cells to absorb circulating amino acids; lack of insulin inhibits absorption.
- Increased potassium uptake – forces cells to absorb serum potassium; lack of insulin inhibits absorption. Insulin's increase in cellular potassium uptake lowers potassium levels in blood. This possible occurs via insulin-induced translocation of the Na+/K+-ATPase to the surface of skeletal muscle cells.[27][28]
- Arterial muscle tone – forces arterial wall muscle to relax, increasing blood flow, especially in microarteries; lack of insulin reduces flow by allowing these muscles to contract.
- Increase in the secretion of hydrochloric acid by parietal cells in the stomach
- Decreased renal sodium excretion.[29]
Degradation
Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment, or it may be degraded by the cell. The two primary sites for insulin clearance are the liver and the kidney. The liver clears most insulin during first-pass transit, whereas the kidney clears most of the insulin in systemic circulation. Degradation normally involves endocytosis of the insulin-receptor complex, followed by the action of insulin-degrading enzyme. An insulin molecule produced endogenously by the pancreatic beta cells is estimated to be degraded within about one hour after its initial release into circulation (insulin half-life ~ 4–6 minutes).[30][31]
Hypoglycemia
Although other cells can use other fuels for a while (most prominently fatty acids), neurons depend on glucose as a source of energy in the nonstarving human. They do not require insulin to absorb glucose, unlike muscle and adipose tissue, and they have very small internal stores of glycogen. Glycogen stored in liver cells (unlike glycogen stored in muscle cells) can be converted to glucose, and released into the blood, when glucose from digestion is low or absent, and the glycerol backbone in triglycerides can also be used to produce blood glucose.
Sufficient lack of glucose and scarcity of these sources of glucose can dramatically make itself manifest in the impaired functioning of the central nervous system: dizziness, speech problems, and even loss of consciousness. Low glucose is known as hypoglycemia or, in cases producing unconsciousness, "hypoglycemic coma" (sometimes termed "insulin shock" from the most common causative agent). Endogenous causes of insulin excess (such as an insulinoma) are very rare, and the overwhelming majority of insulin excess-induced hypoglycemia cases are iatrogenic and usually accidental. A few cases of murder, attempted murder, or suicide using insulin overdoses have been reported, but most insulin shocks appear to be due to errors in dosage of insulin (e.g., 20 units instead of 2) or other unanticipated factors (did not eat as much as anticipated, or exercised more than expected, or unpredicted kinetics of the subcutaneously injected insulin itself).
Possible causes of hypoglycemia include:
- External insulin (usually injected subcutaneously)
- Oral hypoglycemic agents (e.g., any of the sulfonylureas, or similar drugs, which increase insulin release from β-cells in response to a particular blood glucose level)
- Ingestion of low-carbohydrate sugar substitutes in people without diabetes or with type 2 diabetes. Animal studies show these can trigger insulin release, albeit in much smaller quantities than sugar, according to a report in Discover magazine, August 2004, p 18. (This can never be a cause of hypoglycemia in patients with type 1 diabetes, since there is no endogenous insulin production to stimulate.)
Diseases and syndromes
There are several conditions in which insulin disturbance is pathologic:
- Diabetes mellitus – general term referring to all states characterized by hyperglycemia
- Type 1 – autoimmune-mediated destruction of insulin-producing β-cells in the pancreas, resulting in absolute insulin deficiency
- Type 2 – multifactoral syndrome with combined influence of genetic susceptibility and influence of environmental factors, the best known being obesity, age, and physical inactivity, resulting in insulin resistance in cells requiring insulin for glucose absorption. This form of diabetes is strongly inherited.
- Other types of impaired glucose tolerance (see the Diabetes)
- Insulinoma - a tumor of pancreatic β-cells producing excess insulin or reactive hypoglycemia.
- Metabolic syndrome – a poorly understood condition first called Syndrome X by Gerald Reaven, Reaven's Syndrome after Reaven, CHAOS in Australia (from the signs that seem to travel together). It is currently not clear whether these signs have a single, treatable cause, or are the result of body changes leading to type 2 diabetes. It is characterized by elevated blood pressure, dyslipidemia (disturbances in blood cholesterol forms and other blood lipids), and increased waist circumference (at least in populations in much of the developed world). The basic underlying cause may be the insulin resistance that precedes 2 diabetes, which is a diminished capacity for insulin response in some tissues (e.g., muscle, fat). It is common that morbidities, such as essential hypertension, obesity, type 2 diabetes, and cardiovascular disease (CVD) develop.
- Polycystic ovary syndrome – a complex syndrome in women in the reproductive years where anovulation and androgen excess are commonly displayed as hirsutism. In many cases of PCOS, insulin resistance is present.
As a medication
Main article: Insulin therapyBiosynthetic "human" insulin is now manufactured for widespread clinical use using recombinant DNA technology. More recently, researchers have succeeded in introducing the gene for human insulin into plants and in producing insulin in them, to be specific safflower.[32][33] This technique is anticipated to reduce production costs.
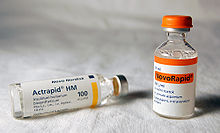
Several of these slightly modified versions of human insulin, while having a clinical effect on blood glucose levels as though they were exact copies, have been designed to have somewhat different absorption or duration of action characteristics. They are usually referred to as "insulin analogues". For instance, the first one available, insulin lispro, does not exhibit a delayed absorption effect found in regular insulin, and begins to have an effect in as little as 15 minutes. Other rapid-acting analogues are NovoRapid and Apidra, with similar profiles. All are rapidly absorbed due to a mutation in the sequence that prevents the insulin analogue from forming dimers and hexamers. Instead, the insulin molecule is a monomer, which is more rapidly absorbed. Using it, therefore, does not require the planning required for other insulins that begin to take effect much later (up to many hours) after administration. Another type is extended-release insulin; the first of these was Lantus (insulin glargine). These have a steady effect for the entire time they are active, without the peak and drop of effect in other insulins; typically, they continue to have an insulin effect for an extended period from 18 to 24 hours. Likewise, another protracted insulin analogue (Levemir) is based on a fatty acid acylation approach. A myristyric acid molecule is attached to this analogue, which in turn associates the insulin molecule to the abundant serum albumin, which in turn extends the effect and reduces the risk of hypoglycemia. Both protracted analogues need to be taken only once-daily, and are very much used in the type 1 diabetes market as the basal insulin. A combination of a rapid acting and a protracted insulin is also available for the patients, making it more likely for them to achieve an insulin profile that mimics that of the body´s own insulin release.
Unlike many medicines, insulin currently cannot be taken orally because, like nearly all other proteins introduced into the gastrointestinal tract, it is reduced to fragments (even single amino acid components), whereupon all activity is lost. There has been some research into ways to protect insulin from the digestive tract, so that it can be administered orally or sublingually. While experimental, several companies now have various formulations in human clinical trials.[34][citation needed]
Insulin is usually taken as subcutaneous injections by single-use syringes with needles, via an insulin pump, or by repeated-use insulin pens with needles.
History
Discovery
In 1869 Paul Langerhans, a medical student in Berlin, was studying the structure of the pancreas under a microscope when he identified some previously unnoticed tissue clumps scattered throughout the bulk of the pancreas. The function of the "little heaps of cells", later known as the islets of Langerhans, was unknown, but Edouard Laguesse later suggested they might produce secretions that play a regulatory role in digestion. Paul Langerhans' son, Archibald, also helped to understand this regulatory role. The term "insulin" origins from insula, the Latin word for islet/island.
In 1889, the Polish-German physician Oscar Minkowski, in collaboration with Joseph von Mering, removed the pancreas from a healthy dog to test its assumed role in digestion. Several days after the dog's pancreas was removed, Minkowski's animal keeper noticed a swarm of flies feeding on the dog's urine. On testing the urine, they found there was sugar in the dog's urine, establishing for the first time a relationship between the pancreas and diabetes. In 1901, another major step was taken by Eugene Opie, when he clearly established the link between the islets of Langerhans and diabetes: "Diabetes mellitus . . . is caused by destruction of the islets of Langerhans and occurs only when these bodies are in part or wholly destroyed." Before his work, the link between the pancreas and diabetes was clear, but not the specific role of the islets.
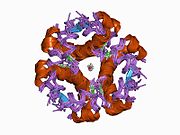
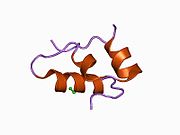
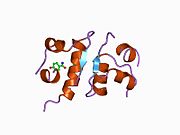
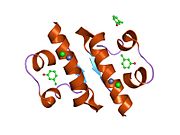
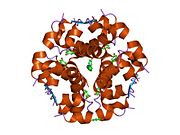
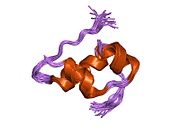
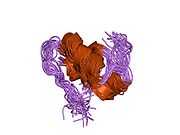
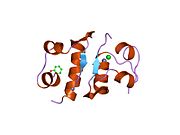
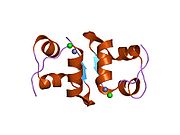
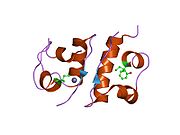
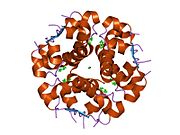
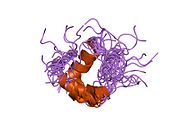
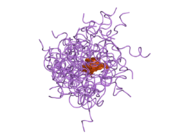
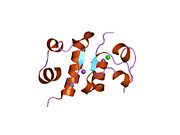
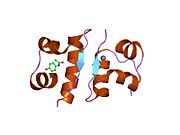
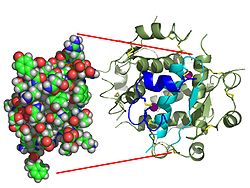 The structure of insulin. The left side is a space-filling model of the insulin monomer, believed to be biologically active. Carbon is green, hydrogen white, oxygen red, and nitrogen blue. On the right side is a ribbon diagram of the insulin hexamer, believed to be the stored form. A monomer unit is highlighted with the A chain in blue and the B chain in cyan. Yellow denotes disulfide bonds, and magenta spheres are zinc ions.
The structure of insulin. The left side is a space-filling model of the insulin monomer, believed to be biologically active. Carbon is green, hydrogen white, oxygen red, and nitrogen blue. On the right side is a ribbon diagram of the insulin hexamer, believed to be the stored form. A monomer unit is highlighted with the A chain in blue and the B chain in cyan. Yellow denotes disulfide bonds, and magenta spheres are zinc ions.
Over the next two decades, several attempts were made to isolate whatever it was the islets produced as a potential treatment. In 1906, George Ludwig Zuelzer was partially successful treating dogs with pancreatic extract, but was unable to continue his work. Between 1911 and 1912, E.L. Scott at the University of Chicago used aqueous pancreatic extracts, and noted "a slight diminution of glycosuria", but was unable to convince his director of his work's value; it was shut down. Israel Kleiner demonstrated similar effects at Rockefeller University in 1915, but his work was interrupted by World War I, and he did not return to it.[35]
Nicolae Paulescu, a Romanian professor of physiology at the University of Medicine and Pharmacy in Bucharest, was the first to isolate insulin, in 1916, which he called at that time, pancrein, by developing an aqueous pancreatic extract which, when injected into a diabetic dog, proved to have a normalizing effect on blood sugar levels. He had to interrupt his experiments because the World War I and in 1921 he wrote four papers about his work carried out in Bucharest and his tests on a diabetic dog. Later that year, he detailed his work by publishing an extensive whitepaper on the effect of the pancreatic extract injected into a diabetic animal, which he called: "Research on the Role of the Pancreas in Food Assimilation" [36][37].
Only 8 months later, the discoveries he published were copied (or, as some say, confirmed) by doctor Frederick Grant Banting and biochemist John James Rickard Macleod, who were later awarded the Nobel prize for the discovery of insulin in 1923, which Paulescu discovered as early as 1916. By the time Banting also isolated insulin, Paulescu already held a patent for his discovery and he was the first to secure the patent rights for his method of manufacturing pancreine/insulin (April 10, 1922, patent no. 6254 (8322) "Pancreina şi procedeul fabricaţiei ei"/"Pancrein and the process of making it", from the Romanian Ministry of Industry and Trade). Moreover, Banting was very familiar with Paulescu’s work, he even used Paulescu’s “Research on the Role of the Pancreas in Food Assimilation” as reference in the paper that brought him the Nobel [38].
Paulescu Controversy
It is often said that the cause for not being recognised as the true discoverer of insulin is because he expressed antisemitic and anti-masonic views. In 2003, following protests from several Jewish organizations, the inauguration of his bust at the Hôtel-Dieu State Hospital in Paris, scheduled for August 27, was cancelled. Also, the French Minister of Health, stated that all his scientific merit must be nullified because of his "brutal inhumanity" of expressing anti-Jewish views. In 2005, the Executive Board of the International Diabetes Federation decided that "the institute does not be associated with Nicolae Paulescu" because of his anti-semitic views and that "there would be no Paulescu Lecture at World Diabetes Congresses should such a request be received”, all his other lectures, or related to him, were banned.
He was also the first individual to use insulin to reduce blood sugar in a mammal, carrying out a series of treatments on diabetics animals and recording its efficacy when injected.[39]
Extraction and Purification in Canada
In October 1920, Canadian Frederick Banting was reading one of Minkowski's papers and concluded that it was the very digestive secretions that Minkowski had originally studied that were breaking down the islet secretion(s), thereby making it impossible to extract successfully. He jotted a note to himself: "Ligate pancreatic ducts of the dog. Keep dogs alive till acini degenerate leaving islets. Try to isolate internal secretion of these and relieve glycosurea."
The idea was the pancreas's internal secretion, which, it was supposed, regulates sugar in the bloodstream, might hold the key to the treatment of diabetes. A surgeon by training, Banting knew certain arteries could be tied off that would lead to atrophy of most of the pancreas, while leaving the islets of Langerhans intact. He theorized a relatively pure extract could be made from the islets once most of the rest of pancreas was gone.
In the spring of 1921, Banting traveled to Toronto to explain his idea to J.J.R. Macleod, who was Professor of Physiology at the University of Toronto, and asked Macleod if he could use his lab space to test the idea. Macleod was initially skeptical, but eventually agreed to let Banting use his lab space while he was on holiday for the summer. He also supplied Banting with ten dogs on which to experiment, and two medical students, Charles Best and Clark Noble, to use as lab assistants, before leaving for Scotland. Since Banting required only one lab assistant, Best and Noble flipped a coin to see which would assist Banting for the first half of the summer. Best won the coin toss, and took the first shift as Banting's assistant. Loss of the coin toss may have proved unfortunate for Noble, given that Banting decided to keep Best for the entire summer, and eventually shared half his Nobel Prize money and a large part of the credit for the discovery of insulin with the winner of the toss. Had Noble won the toss, his career might have taken a different path.[40] Banting's method was to tie a ligature around the pancreatic duct; when examined several weeks later, the pancreatic digestive cells had died and been absorbed by the immune system, leaving thousands of islets. They then isolated an extract from these islets, producing what they called "isletin" (what we now know as insulin), and tested this extract on the dogs starting July 27.[41] Banting and Best were then able to keep a pancreatectomized dog named Alpha alive for the rest of the summer by injecting her with the crude extract they had prepared. Removal of the pancreas in test animals essentially mimics diabetes, leading to elevated blood glucose levels. Alpha was able to remain alive because the extracts, containing isletin, were able to lower her blood glucose levels.
Banting and Best presented their results to Macleod on his return to Toronto in the fall of 1921, but Macleod pointed out flaws with the experimental design, and suggested the experiments be repeated with more dogs and better equipment. He then supplied Banting and Best with a better laboratory, and began paying Banting a salary from his research grants. Several weeks later, the second round of experiments clearly was also a success; and Macleod helped publish their results privately in Toronto that November. However, they needed six weeks to extract the isletin, which forced considerable delays. Banting suggested they try to use fetal calf pancreas, which had not yet developed digestive glands; he was relieved to find this method worked well. With the supply problem solved, the next major effort was to purify the extract. In December 1921, Macleod invited the biochemist James Collip to help with this task, and, within a month, the team felt ready for a clinical test.
On January 11, 1922, Leonard Thompson, a 14-year-old diabetic who lay dying at the Toronto General Hospital, was given the first injection of insulin. However, the extract was so impure, Thompson suffered a severe allergic reaction, and further injections were canceled. Over the next 12 days, Collip worked day and night to improve the ox-pancreas extract, and a second dose was injected on January 23. This was completely successful, not only in having no obvious side-effects, but also in completely eliminating the glycosuria sign of diabetes. The first American patient was Elizabeth Hughes Gossett, the daughter of the governor of New York.[42] The first patient treated in the U.S. was future woodcut artist James D. Havens; Dr. John Ralston Williams imported insulin from Toronto to Rochester, New York, to treat Havens.[43]
Children dying from diabetic ketoacidosis were kept in large wards, often with 50 or more patients in a ward, mostly comatose. Grieving family members were often in attendance, awaiting the (until then, inevitable) death.
In one of medicine's more dramatic moments, Banting, Best, and Collip went from bed to bed, injecting an entire ward with the new purified extract. Before they had reached the last dying child, the first few were awakening from their coma, to the joyous exclamations of their families.[44]
Banting and Best never worked well with Collip, regarding him as something of an interloper, and Collip left the project soon after.
Over the spring of 1922, Best managed to improve his techniques to the point where large quantities of insulin could be extracted on demand, but the preparation remained impure. The drug firm Eli Lilly and Company had offered assistance not long after the first publications in 1921, and they took Lilly up on the offer in April. In November, Lilly made a major breakthrough and were able to produce large quantities of highly refined insulin. Insulin was offered for sale shortly thereafter.
Synthesis
Purified animal-sourced insulin was the only type of insulin available to diabetics until genetic advances occurred later with medical research. The amino acid structure of insulin was characterized in the 1950s,[45] and the first synthetic insulin was produced simultaneously in the labs of Panayotis Katsoyannis at the University of Pittsburgh and Helmut Zahn at RWTH Aachen University in the early 1960s.[46][47]
The first genetically-engineered, synthetic "human" insulin was produced in a laboratory in 1977 by Herbert Boyer using E. coli.[48][49] Partnering with Genentech founded by Boyer, Eli Lilly and Company went on in 1982 to sell the first commercially available biosynthetic human insulin under the brand name Humulin.[49] The vast majority of insulin currently used worldwide is now biosynthetic recombinant "human" insulin or its analogues.
Nobel Prizes
The Nobel Prize committee in 1923 credited the practical extraction of insulin to a team at the University of Toronto and awarded the Nobel Prize to two men: Frederick Banting and J.J.R. Macleod.[50]. They were awarded the Nobel Prize in Physiology or Medicine in 1923 for the discovery of insulin. Banting, insulted that Best was not mentioned, shared his prize with him, and Macleod immediately shared his with James Collip. The patent for insulin was sold to the University of Toronto for one half-dollar.
While Paulescu's pioneering work, which had been cited in Banting and Rickard's prize-winning research, was being completely ignored by the Nobel prize committee, Professor Ian Murray was particularly active in working to correct the historical wrong against Paulescu. Murray was a professor of physiology at the Anderson College of Medicine in Glasgow, Scotland, the head of the department of Metabolic Diseases at a leading Glasgow hospital, vice-president of the British Association of Diabetes, and a founding member of the International Diabetes Federation. In an article for a 1971 issue of the Journal of the History of Medicine and Allied Sciences, Murray wrote:
"Insufficient recognition has been given to Paulesco, the distinguished Roumanian scientist, who at the time when the Toronto team were commencing their research had already succeeded in extracting the antidiabetic hormone of the pancreas and proving its efficacy in reducing the hyperglycaemia in diabetic dogs."
Furthermore, Murray reported:
"In a recent private communication Professor Tiselius, head of the Nobel Institute, has expressed his personal opinion that Paulesco was equally worthy of the award in 1923."[39]
The primary structure of insulin was determined by British molecular biologist Frederick Sanger.[45] It was the first protein to have its sequence be determined. He was awarded the 1958 Nobel Prize in Chemistry for this work.
In 1969, after decades of work, Dorothy Crowfoot Hodgkin determined the spatial conformation of the molecule, the so-called tertiary structure, by means of X-ray diffraction studies. She had been awarded a Nobel Prize in Chemistry in 1964 for the development of crystallography.
Rosalyn Sussman Yalow received the 1977 Nobel Prize in Medicine for the development of the radioimmunoassay for insulin.
See also
- Insulin analog
- Anatomy and physiolology
- Pancreas
- Islets of Langerhans
- Endocrinology
- Leptin (The only other known adiposity signal besides insulin).
- Forms of diabetes mellitus
- Treatment
- Other medical / diagnostic uses
References
- ^ PDB 1ai0; Chang X, Jorgensen AM, Bardrum P, Led JJ (August 1997). "Solution structures of the R6 human insulin hexamer,". Biochemistry 36 (31): 9409–22. doi:10.1021/bi9631069. PMID 9235985.
- ^ Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. (November 2004). "Intranasal insulin improves memory in humans.". Psychoneuroendocrinology 29 (10): 1326–34. doi:10.1016/j.psyneuen.2004.04.003. PMID 15288712.
- ^ Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, Hallschmid M. (2010). "Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men.". Diabetes 60 (1): 114–118. doi:10.2337/db10-0329. PMC 3012162. PMID 20876713. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3012162.
- ^ a b "Entrez Gene: INS insulin". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=3630.
- ^ Bell GI, Pictet RL, Rutter WJ, Cordell B, Tischer E, Goodman HM (March 1980). "Sequence of the human insulin gene". Nature 284 (5751): 26–32. doi:10.1038/284026a0. PMID 6243748.
- ^ Melloul D, Marshak S, Cerasi E (2002). "Regulation of insulin gene transcription". Diabetologia 45 (3): 309–26. doi:10.1007/s00125-001-0728-y. PMID 11914736.
- ^ Jang WG, Kim EJ, Park KG, Park YB, Choi HS, Kim HJ, Kim YD, Kim KS, Lee KU, Lee IK (2007). "Glucocorticoid receptor mediated repression of human insulin gene expression is regulated by PGC-1alpha". Biochem. Biophys. Res. Commun. 352 (3): 716–21. doi:10.1016/j.bbrc.2006.11.074. PMID 17150186.
- ^ Sanger F, Tuppy H (September 1951). "The amino-acid sequence in the phenylalanyl chain of insulin. I. The identification of lower peptides from partial hydrolysates". Biochem. J. 49 (4): 463–81. PMC 1197535. PMID 14886310. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1197535.; Sanger F, Tuppy H (September 1951). "The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates". Biochem. J. 49 (4): 481–90. PMC 1197536. PMID 14886311. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1197536.; Sanger F, Thompson EO (February 1953). "The amino-acid sequence in the glycyl chain of insulin. I. The identification of lower peptides from partial hydrolysates". Biochem. J. 53 (3): 353–66. PMC 1198157. PMID 13032078. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1198157.; Sanger F, Thompson EO (February 1953). "The amino-acid sequence in the glycyl chain of insulin. II. The investigation of peptides from enzymic hydrolysates". Biochem. J. 53 (3): 366–74. PMC 1198158. PMID 13032079. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1198158.
- ^ Katsoyannis PG, Fukuda K, Tometsko A, Suzuki K, Tilak M, Panayotis G.; Fukuda, Kouhei; Tometsko, Andrew; Suzuki, Kenji; Tilak, Manohar (1964). "Insulin Peptides. X. The Synthesis of the B-Chain of Insulin and Its Combination with Natural or Synthetis A-Chin to Generate Insulin Activity". Journal of the American Chemical Society 86 (5): 930–932. doi:10.1021/ja01059a043.
- ^ Kung YT, Du YC, Huang WT, Chen CC, Ke LT (November 1965). "Total synthesis of crystalline bovine insulin". Sci. Sin. 14 (11): 1710–6. PMID 5881570.
- ^ Marglin B, Merrifield RB (1966). "The Synthesis of Bovine Insulin by the Solid Phase Method". Journal of the American Chemical Society 88 (21): 5051–5052. doi:10.1021/ja00973a068. PMID 5978833.
- ^ Dunn MF (August 2005). "Zinc-ligand interactions modulate assembly and stability of the insulin hexamer -- a review". Biometals 18 (4): 295–303. doi:10.1007/s10534-005-3685-y. PMID 16158220.
- ^ Ivanova MI, Sievers SA, Sawaya MR, Wall JS, Eisenberg D (November 2009). "Molecular basis for insulin fibril assembly". Proc. Natl. Acad. Sci. U.S.A. 106 (45): 18990–5. doi:10.1073/pnas.0910080106. PMC 2776439. PMID 19864624. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2776439.
- ^ Steiner DF, Oyer PE (February 1967). "The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma". Proc. Natl. Acad. Sci. U.S.A. 57 (2): 473–480. doi:10.1073/pnas.57.2.473. PMC 335530. PMID 16591494. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=335530.
- ^ Gustin N (2005-03-07). "Researchers discover link between insulin and Alzheimer's". EurekAlert!. American Association for the Advancement of Science. http://www.eurekalert.org/pub_releases/2005-03/l-rdl030205.php. Retrieved 2009-01-01.
- ^ de la Monte SM, Wands JR (February 2005). "Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease". J. Alzheimers Dis. 7 (1): 45–61. PMID 15750214. http://iospress.metapress.com/openurl.asp?genre=article&issn=1387-2877&volume=7&issue=1&spage=45.
- ^ Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (February 2005). "Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes?". J. Alzheimers Dis. 7 (1): 63–80. PMID 15750215. http://iospress.metapress.com/openurl.asp?genre=article&issn=1387-2877&volume=7&issue=1&spage=63.
- ^ a b c d e Hellman B, Gylfe E, Grapengiesser E, Dansk H, Salehi A (2007). "[Insulin oscillations--clinically important rhythm. Antidiabetics should increase the pulsative component of the insulin release]" (in Swedish). Lakartidningen 104 (32–33): 2236–9. PMID 17822201.
- ^ A Dictionary of Units of Measurement By Russ Rowlett, the University of North Carolina at Chapel Hill. June 13, 2001
- ^ Iwase, H.; Kobayashi, M.; Nakajima, M.; Takatori, T. (2001). "The ratio of insulin to C-peptide can be used to make a forensic diagnosis of exogenous insulin overdosage". Forensic science international 115 (1–2): 123–127. doi:10.1016/S0379-0738(00)00298-X. PMID 11056282.
- ^ Xianjun Fang, Shuang Xing Yu, Yiling Lu, Robert C. Bast, Jr. James R. Woodgett, and Gordon B. Mills (2000). "Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A". Proceedings of the National Academy of Sciences of the United States of America 97 (22): 11960–11965. doi:10.1073/pnas.220413597. PMC 17277. PMID 11035810. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=17277.
- ^ Edward J McManus, Kei Sakamoto, Laura J Armit, Leah Ronaldson, Natalia Shpiro, Rodolfo Marquez and Dario R Alessi (2005). "Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis". The EMBO Journal 24 (8): 1571–1583. doi:10.1038/sj.emboj.7600633. PMC 1142569. PMID 15791206. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1142569.
- ^ Painter PC, Mosher LE, Rhoads C (July 1982). "Low-frequency modes in the Raman spectra of proteins". Biopolymers 21 (7): 1469–72. doi:10.1002/bip.360210715. PMID 7115900.
- ^ Chou KC (December 1983). "Identification of low-frequency modes in protein molecules". Biochem. J. 215 (3): 465–9. PMC 1152424. PMID 6362659. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1152424.
- ^ Chou KC (May 1988). "Low-frequency collective motion in biomacromolecules and its biological functions". Biophys. Chem. 30 (1): 3–48. PMID 3046672.
- ^ Bergamini E, Cavallini G, Donati A, Gori Z (October 2007). "The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction". Ann. N. Y. Acad. Sci. 1114: 69–78. doi:10.1196/annals.1396.020. PMID 17934054.
- ^ Benziane B, Chibalin AV (2008). "Frontiers: skeletal muscle sodium pump regulation: a translocation paradigm". American Journal of Physiology. Endocrinology and Metabolism 295 (3): E553-8. doi:10.1152/ajpendo.90261.2008. PMID 18430962. http://ajpendo.physiology.org/content/295/3/E553.long.
- ^ Clausen T (2008). "Regulatory role of translocation of Na+-K+ pumps in skeletal muscle: hypothesis or reality?". American Journal of Physiology. Endocrinology and Metabolism 295 (3): E727-8. doi:10.1152/ajpendo.90494.2008. PMID 18775888. http://ajpendo.physiology.org/content/295/3/E727.long.
- ^ Gupta AK, Clark RV, Kirchner KA (1992). "Effects of insulin on renal sodium excretion". Hypertension 19 (1 Suppl): 178–182. PMID 1730458.
- ^ William C. Duckworth, Robert G. Bennett and Frederick G. Hamel (1998). "Insulin Degradation: Progress and Potential". Endocrine Reviews 19 (5): 608–624. doi:10.1210/er.19.5.608. PMID 9793760. http://edrv.endojournals.org/cgi/content/full/19/5/608#F1.
- ^ Palmer, BF et. al. 2010. "Carbohydrate and insulin metabolism in chronic kidney disease." UpToDate Online, accessed Nov. 13, 2010.
- ^ From SemBiosys, A New Kind Of Insulin INSIDE WALL STREET By Gene G. Marcial(AUGUST 13, 2007)
- ^ http://www.i-sis.org.uk/gmSaffloweHumanPro-Insulin.php
- ^ NDTV Profit December 11, 2009-Biocon May Launch Oral Insulin
- ^ The American Institute of Nutrition (1967). "Proceedings of the Thirty-first Annual Meeting of the American Institute of Nutrition" (PDF). Journal of Nutrition 92: 509. http://jn.nutrition.org/cgi/reprint/92/4/507.pdf.
- ^ Paulesco, N.C. (1921, August 31), "Recherche sur le rôle du pancréas dans l'assimilation nutritive.", Archives Internationales de Physiologie 17: 85–103
- ^ Lestradet, H. (1997), "Le 75e anniversaire de la découverte de l’insuline.", Diabetes & Metabolism 23 (1): 112, http://www.em-consulte.com/en/article/79613
- ^ Banting FG, Best CH. (1922), "The internal secretion of pancreas.", J Lab Clin Med 7: 251–266, http://www.biology.buffalo.edu/courses/bio130/medler/banting.pdf
- ^ a b Ian Murray (1971). "Paulesco and the Isolation of Insulin" (PDF). Journal of the History of Medicine and Allied Sciences 26 (2): 150–157. PMID 4930788. http://jhmas.oxfordjournals.org/cgi/reprint/XXVI/2/150.pdf.
- ^ Wright JR (December 2002). "Almost famous: E. Clark Noble, the common thread in the discovery of insulin and vinblastine". CMAJ 167 (12): 1391–6. PMC 137361. PMID 12473641. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=137361.
- ^ Krishnamurthy, Kalayya (2002). Pioneers in scientific discoveries. Mittal Publications. p. 266. ISBN 9788170998440. http://books.google.com/books?id=dAXYzzDL_9oC&pg=PA266. Retrieved 26 July 2011.
- ^ Abigail Zuger (October 4, 2010). "Rediscovering the First Miracle Drug". New York Times. http://www.nytimes.com/2010/10/05/health/05insulin.html?_r=1&hp=&pagewanted=all. Retrieved 2010-10-06. "Elizabeth Hughes was a cheerful, pretty little girl, five feet tall, with straight brown hair and a consuming interest in birds. On Dr. Allen’s diet her weight fell to 65 pounds, then 52 pounds, and then, after an episode of diarrhea that almost killed her in the spring of 1922, 45 pounds. By then she had survived three years, far longer than expected. And then her mother heard the news: Insulin had finally been isolated in Canada."
- ^ Marcotte, Bob (November 22, 2010). "Rochester's John Williams a man of scientific talents". Democrat and Chronicle. Gannett Company (Rochester, New York): pp. 1B, 4B. Archived from the original on November 22, 2010. http://www.webcitation.org/5uRSurOlI. Retrieved November 22, 2010.
- ^ Medical News Today-Discovery of Insulin
- ^ a b[citation needed]
- ^ Goro, Fritz (1964-05-08). "First Man-made Protein in History". Life (New York, NY: Time, Inc.) 56 (1): 47. doi:10.1080/15216540310001659029. PMID 14992380. http://books.google.com/?id=lkEEAAAAMBAJ&lpg=PA47&vq=insulin&pg=PA47#v=onepage&q=insulin. Retrieved 2009-11-02.
- ^ Federwisch, Matthias; Dieken, Markus Leyck; De Meyts, Pierre, eds (2002). Insulin & Related Proteins – Structure to Function and Pharmacology. Dordrecht, Netherlands: Kluwer Academic Publishers. pp. ix. ISBN 1-4020-0655-1. http://books.google.com/?id=Ula72_FSwy8C&lpg=PP11&dq=Panayotis%20Katsoyannis&pg=PP11#v=onepage&q=Panayotis%20Katsoyannis. Retrieved 2009-11-02.
- ^ "First Successful Laboratory Production of Human Insulin Announced". News Release. Genentech. 1978-09-06. http://www.gene.com/gene/news/press-releases/display.do?method=detail&id=4160. Retrieved 2009-11-03.
- ^ a b Tof I (1994). "Recombinant DNA technology in the synthesis of human insulin". Little Tree Publishing. http://www.littletree.com.au/dna.htm. Retrieved 2009-11-03.
- ^ "The Nobel Prize in Physiology or Medicine 1923". The Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1923/.
Further reading
- Reaven, Gerald M.; Ami Laws (ed.) (1999--04-15). Insulin Resistance: The Metabolic Syndrome X (1st ed.). Totowa, New Jersey: Humana Press. doi:10.1226/0896035883. ISBN 0-89603-588-3.
- Leahy, Jack L.; William T. Cefalu (ed.) (2002-03-22). Insulin Therapy (1st ed.). New York: Marcel Dekker. ISBN 0-8247-0711-7.
- Kumar, Sudhesh; Stephen O'Rahilly (ed.) (2005-01-14). Insulin Resistance: Insulin Action and Its Disturbances in Disease. Chichester, England: Wiley. ISBN 0-470-85008-6.
- Ehrlich, Ann; Carol L. Schroeder (2000-06-16). Medical Terminology for Health Professions (4th ed.). Thomson Delmar Learning. ISBN 0-7668-1297-9.
- Draznin, Boris; Derek LeRoith (September 1994). Molecular Biology of Diabetes: Autoimmunity and Genetics; Insulin Synthesis and Secretion. Totowa, New Jersey: Humana Press. doi:10.1226/0896032868. ISBN 0-89603-286-8.
- Famous Canadian Physicians: Sir Frederick Banting at Library and Archives Canada
- McKeage K, Goa KL (2001). "Insulin glargine: a review of its therapeutic use as a long-acting agent for the management of type 1 and 2 diabetes mellitus". Drugs 61 (11): 1599–624. doi:10.2165/00003495-200161110-00007. PMID 11577797.
External links
- The Insulin Protein
- Inspired by Insulin article by parent of a diabetic child
- Frederick Sanger, Nobel Prize for sequencing Insulin Freeview video with John Sanger and John Walker by the Vega Science Trust.
- Insulin: entry from protein databank
- The History of Insulin
- Insulin Lispro
- CBC Digital Archives - Banting, Best, Macleod, Collip: Chasing a Cure for Diabetes
- GeneReviews/NCBI/NIH/UW entry on Permanent Neonatal Diabetes Mellitus
- Cosmos Magazine: Insulin mystery cracked after 20 years
- National Diabetes Information Clearinghouse
- Discovery and Early Development of Insulin, 1920–1925
- Secretion of Insulin and Glucagon
- Insulin Types Comparison Chart
- Insulin hormone dosage and side effects
- The True Discoverer of Insulin - Nicolae Paulescu
- Insulin signaling pathway
- Molecular Physiology of Signalling Proteins
- Animations of insulin's action in the body at AboutKidsHealth.ca
PDB gallery 1ai0: R6 HUMAN INSULIN HEXAMER (NON-SYMMETRIC), NMR, 10 STRUCTURES1aiy: R6 HUMAN INSULIN HEXAMER (SYMMETRIC), NMR, 10 STRUCTURES1aph: CONFORMATIONAL CHANGES IN CUBIC INSULIN CRYSTALS IN THE PH RANGE 7-111b17: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 5.00 COORDINATES)1b18: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 5.53 COORDINATES)1b19: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 5.80 COORDINATES)1b2a: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 6.00 COORDINATES)1b2b: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 6.16 COORDINATES)1b2c: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 6.26 COORDINATES)1b2d: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 6.35 COORDINATES)1b2e: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 6.50 COORDINATES)1b2f: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 6.98 COORDINATES)1b2g: PH AFFECTS GLU B13 SWITCHING AND SULFATE BINDING IN CUBIC INSULIN CRYSTALS (PH 9.00 COORDINATES)1b9e: HUMAN INSULIN MUTANT SERB9GLU1ben: INSULIN COMPLEXED WITH 4-HYDROXYBENZAMIDE1bph: CONFORMATIONAL CHANGES IN CUBIC INSULIN CRYSTALS IN THE PH RANGE 7-111cph: CONFORMATIONAL CHANGES IN CUBIC INSULIN CRYSTALS IN THE PH RANGE 7-111dph: CONFORMATIONAL CHANGES IN CUBIC INSULIN CRYSTALS IN THE PH RANGE 7-111ev3: Structure of the rhombohedral form of the M-cresol/insulin R6 hexamer1ev6: Structure of the monoclinic form of the M-cresol/insulin R6 hexamer1evr: The structure of the resorcinol/insulin R6 hexamer1fu2: FIRST PROTEIN STRUCTURE DETERMINED FROM X-RAY POWDER DIFFRACTION DATA1fub: FIRST PROTEIN STRUCTURE DETERMINED FROM X-RAY POWDER DIFFRACTION DATA1g7a: 1.2 A structure of T3R3 human insulin at 100 K1g7b: 1.3 A STRUCTURE OF T3R3 HUMAN INSULIN AT 100 K1guj: INSULIN AT PH 2: STRUCTURAL ANALYSIS OF THE CONDITIONS PROMOTING INSULIN FIBRE FORMATION.1hiq: PARADOXICAL STRUCTURE AND FUNCTION IN A MUTANT HUMAN INSULIN ASSOCIATED WITH DIABETES MELLITUS1hit: RECEPTOR BINDING REDEFINED BY A STRUCTURAL SWITCH IN A MUTANT HUMAN INSULIN1hls: NMR STRUCTURE OF THE HUMAN INSULIN-HIS(B16)1htv: CRYSTAL STRUCTURE OF DESTRIPEPTIDE (B28-B30) INSULIN1iza: ROLE OF B13 GLU IN INSULIN ASSEMBLY: THE HEXAMER STRUCTURE OF RECOMBINANT MUTANT (B13 GLU-> GLN) INSULIN1izb: ROLE OF B13 GLU IN INSULIN ASSEMBLY: THE HEXAMER STRUCTURE OF RECOMBINANT MUTANT (B13 GLU-> GLN) INSULIN1j73: Crystal structure of an unstable insulin analog with native activity.1jca: Non-standard Design of Unstable Insulin Analogues with Enhanced Activity1jco: Solution structure of the monomeric [Thr(B27)->Pro,Pro(B28)->Thr] insulin mutant (PT insulin)1lph: LYS(B28)PRO(B29)-HUMAN INSULIN1m5a: Crystal Structure of 2-Co(2+)-Insulin at 1.2A Resolution1mhi: THREE-DIMENSIONAL SOLUTION STRUCTURE OF AN INSULIN DIMER. A STUDY OF THE B9(ASP) MUTANT OF HUMAN INSULIN USING NUCLEAR MAGNETIC RESONANCE DISTANCE GEOMETRY AND RESTRAINED MOLECULAR DYNAMICS1mhj: SOLUTION STRUCTURE OF THE SUPERACTIVE MONOMERIC DES-[PHE(B25)] HUMAN INSULIN MUTANT. ELUCIDATION OF THE STRUCTURAL BASIS FOR THE MONOMERIZATION OF THE DES-[PHE(B25)] INSULIN AND THE DIMERIZATION OF NATIVE INSULIN1mpj: X-RAY CRYSTALLOGRAPHIC STUDIES ON HEXAMERIC INSULINS IN THE PRESENCE OF HELIX-STABILIZING AGENTS, THIOCYANATE, METHYLPARABEN AND PHENOL1mso: T6 Human Insulin at 1.0 A Resolution1os3: Dehydrated T6 human insulin at 100 K1os4: Dehydrated T6 human insulin at 295 K1q4v: CRYSTAL STRUCTURE OF ALLO-ILEA2-INSULIN, AN INACTIVE CHIRAL ANALOGUE: IMPLICATIONS FOR THE MECHANISM OF RECEPTOR1qiy: HUMAN INSULIN HEXAMERS WITH CHAIN B HIS MUTATED TO TYR COMPLEXED WITH PHENOL1qiz: HUMAN INSULIN HEXAMERS WITH CHAIN B HIS MUTATED TO TYR COMPLEXED WITH RESORCINOL1qj0: HUMAN INSULIN HEXAMERS WITH CHAIN B HIS MUTATED TO TYR1rwe: Enhancing the activity of insulin at receptor edge: crystal structure and photo-cross-linking of A8 analogues1sf1: NMR STRUCTURE OF HUMAN INSULIN under Amyloidogenic Condition, 15 STRUCTURES1t0c: Solution Structure of Human Proinsulin C-Peptide1trz: CRYSTALLOGRAPHIC EVIDENCE FOR DUAL COORDINATION AROUND ZINC IN THE T3R3 HUMAN INSULIN HEXAMER1tyl: THE STRUCTURE OF A COMPLEX OF HEXAMERIC INSULIN AND 4'-HYDROXYACETANILIDE1tym: THE STRUCTURE OF A COMPLEX OF HEXAMERIC INSULIN AND 4'-HYDROXYACETANILIDE1uz9: CRYSTALLOGRAPHIC AND SOLUTION STUDIES OF N-LITHOCHOLYL INSULIN: A NEW GENERATION OF PROLONGED-ACTING INSULINS.1w8p: STRUCTURAL PROPERTIES OF THE B25TYR-NME-B26PHE INSULIN MUTANT.1wav: CRYSTAL STRUCTURE OF FORM B MONOCLINIC CRYSTAL OF INSULIN1xda: STRUCTURE OF INSULIN1xgl: HUMAN INSULIN DISULFIDE ISOMER, NMR, 10 STRUCTURES1xw7: Diabetes-Associated Mutations in Human Insulin: Crystal Structure and Photo-Cross-Linking Studies of A-Chain Variant Insulin Wakayama1zeg: STRUCTURE OF B28 ASP INSULIN IN COMPLEX WITH PHENOL1zeh: STRUCTURE OF INSULIN1zni: INSULIN1znj: INSULIN, MONOCLINIC CRYSTAL FORM2a3g: The structure of T6 bovine insulin2aiy: R6 HUMAN INSULIN HEXAMER (SYMMETRIC), NMR, 20 STRUCTURES2bn1: INSULIN AFTER A HIGH DOSE X-RAY BURN2bn3: INSULIN BEFORE A HIGH DOSE X-RAY BURN2c8q: INSULINE(1SEC) AND UV LASER EXCITED FLUORESCENCE2c8r: INSULINE(60SEC) AND UV LASER EXCITED FLUORESCENCE2g4m: Insulin collected at 2.0 A wavelength2g54: Crystal structure of Zn-bound human insulin-degrading enzyme in complex with insulin B chain2g56: crystal structure of human insulin-degrading enzyme in complex with insulin B chain2hiu: NMR STRUCTURE OF HUMAN INSULIN IN 20% ACETIC ACID, ZINC-FREE, 10 STRUCTURES2ins: THE STRUCTURE OF DES-PHE B1 BOVINE INSULIN2omg: Structure of human insulin cocrystallized with protamine and urea2omh: Structure of human insulin cocrystallized with ARG-12 peptide in presence of urea2omi: Structure of human insulin cocrystallized with protamine2tci: X-RAY CRYSTALLOGRAPHIC STUDIES ON HEXAMERIC INSULINS IN THE PRESENCE OF HELIX-STABILIZING AGENTS, THIOCYANATE, METHYLPARABEN AND PHENOL3aiy: R6 HUMAN INSULIN HEXAMER (SYMMETRIC), NMR, REFINED AVERAGE STRUCTURE3ins: STRUCTURE OF INSULIN. RESULTS OF JOINT NEUTRON AND X-RAY REFINEMENT3mth: X-RAY CRYSTALLOGRAPHIC STUDIES ON HEXAMERIC INSULINS IN THE PRESENCE OF HELIX-STABILIZING AGENTS, THIOCYANATE, METHYLPARABEN AND PHENOL4aiy: R6 HUMAN INSULIN HEXAMER (SYMMETRIC), NMR, 'GREEN' SUBSTATE, AVERAGE STRUCTURE4ins: THE STRUCTURE OF 2ZN PIG INSULIN CRYSTALS AT 1.5 ANGSTROMS RESOLUTION5aiy: R6 HUMAN INSULIN HEXAMER (SYMMETRIC), NMR, 'RED' SUBSTATE, AVERAGE STRUCTURE6ins: X-RAY ANALYSIS OF THE SINGLE CHAIN /B29-A1$ PEPTIDE-LINKED INSULIN MOLECULE. A COMPLETELY INACTIVE ANALOGUE7ins: STRUCTURE OF PORCINE INSULIN COCRYSTALLIZED WITH CLUPEINE Z9ins: MONOVALENT CATION BINDING IN CUBIC INSULIN CRYSTALSEndocrine system: hormones (Peptide hormones · Steroid hormones) Endocrine
glandsTestis: testosterone · AMH · inhibin
Ovary: estradiol · progesterone · activin and inhibin · relaxin (pregnancy)
Placenta: hCG · HPL · estrogen · progesteroneIslet-Acinar
AxisPancreas: glucagon · insulin · amylin · somatostatin · pancreatic polypeptide
Pineal gland: melatoninNon-end.
glandsThymus: Thymosin (Thymosin α1, Thymosin beta) · Thymopoietin · Thymulin
Digestive system: Stomach: gastrin · ghrelin · Duodenum: CCK · GIP · secretin · motilin · VIP · Ileum: enteroglucagon · peptide YY · Liver/other: Insulin-like growth factor (IGF-1, IGF-2)
Adipose tissue: leptin · adiponectin · resistin
Kidney: JGA (renin) · peritubular cells (EPO) · calcitriol · prostaglandin
Heart: Natriuretic peptide (ANP, BNP)Categories:- Human proteins
- Eli Lilly and Company
- Recombinant proteins
- Peptide hormones
- Human hormones
- Hormones of glucose metabolism
- Pancreatic hormones
- Insulin therapies
- Animal products
- Tumor markers
Wikimedia Foundation. 2010.