- Mannose
-
Mannose 
Identifiers CAS number 31103-86-3 
PubChem 18950 UNII PHA4727WTP 
MeSH Mannose ChEMBL CHEMBL469448 
Properties Molecular formula C6H12O6 Molar mass 180.156 g mol-1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics.
Contents
Structure
Two of the cyclic mannose isomers possess a pyranose (six-membered) ring, while the other two possess a furanose (five-membered) ring.
D-Mannose isomers Skeletal formula Haworth projection 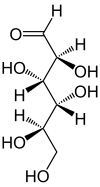
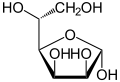
α-D-Mannofuranose
<1 %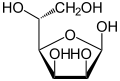
β-D-Mannofuranose
<1 %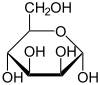
α-D-Mannopyranose
67 %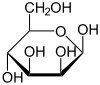
β-D-Mannopyranose
33 %Metabolism
Mannose is not well metabolized in humans.[1] Therefore, it does not significantly enter the carbohydrate metabolism when taken orally, and although traces of exogeneously introduced mannose have been detected in all body tissues, using radioactive markers, in a well hydrated mammal, 90% of mannose ingested is excreted unconverted into the urine within 30 – 60 minutes, with 99% of the remainder being excreted within the following 8 hours. There is no significant increase in blood-glucose levels during this time.
Mannose is present in numerous glycoconjugates including N-linked glycosylation of proteins. C-mannosylation is also abundant and can be found in collagen-like regions. Mannose is a C-2 epimer of glucose and displays a 4C1 pucker in the solution ring form.
Recombinant proteins produced in yeast may be subject to mannose addition in patterns different from those used by mammalian cells.[2] This difference in recombinant proteins from those normally produced in mammalian organisms may influence the effectiveness of vaccines.
Formation
Mannose can be formed by the oxidation of mannitol.
It can also be formed from glucose in the Lobry-de Bruyn-van Ekenstein transformation
D-mannose is sold as a naturopathic remedy for urinary tract infections, and it is claimed to work through the disruption of adherence of bacteria in the urinary tract.
Etymology
The root of both "mannose" and "mannitol" is manna, which the Bible records as the food supplied to the Israelites during their journey through the Sinai Peninsula. Manna is a sweet secretion of several trees and shrubs, such as Fraxinus ornus.
Configuration
Mannose differs from glucose by inversion of the C-2 chiral center. This apparently simple change leads to the drastically different chemistry of the two hexoses, as it does the remaining six aldohexoses.
See also
References
- ^ Direct utilization of mannose for mammalian glycoprotein biosynthesis. Oxford Journals, Life Sciences, Glycobiology, Volume 8, Number 3 Pp. 285-295.
- ^ Vlahopoulos S, Gritzapis AD, Perez SA, Cacoullos N, Papamichail M, Baxevanis CN. Mannose addition by yeast Pichia Pastoris on recombinant HER-2 protein inhibits recognition by the monoclonal antibody herceptin. Vaccine. 2009 Jul 23;27(34):4704-8. Epub 2009 Jun 9.PMID: 19520203.
External links
Types of carbohydrates General: Geometry Monosaccharides Aldodiose (Glycolaldehyde)Ketopentose (Ribulose, Xylulose)
Aldopentose (Ribose, Arabinose, Xylose, Lyxose)
Deoxy sugar (Deoxyribose)Ketoheptose (Sedoheptulose, Mannoheptulose)>7Multiple Other oligosaccharidesGlucose/Glucan: Glycogen · Starch (Amylose, Amylopectin) · Cellulose · Dextrin/Dextran · Beta-glucan (Zymosan, Lentinan, Sizofiran) · Maltodextrin
Fructose/Fructan: Inulin · Levan beta 2→6
Mannose/Mannan
N-Acetylglucosamine: Chitinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i -
Categories:
Wikimedia Foundation. 2010.

