- Maltose
-
Maltose 
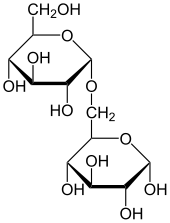 2-(hydroxymethyl)-6-[4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyox ane-3,4,5-triolOther names4-O-α-D-Glucopyranosyl-D-glucose
2-(hydroxymethyl)-6-[4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyox ane-3,4,5-triolOther names4-O-α-D-Glucopyranosyl-D-glucose
Isomaltose
6-O-α-D-Glucopyranosyl-D-glucoseIdentifiers CAS number 69-79-4 
PubChem 6255, 439193 (isomaltose) ChemSpider 388329 α-maltose  , 6019 β-maltose
, 6019 β-maltoseUNII 66Y63L379N 
EC-number 200-716-5 ChEBI CHEBI:17306 
ChEMBL CHEMBL1234209 
Jmol-3D images Image 1 - O([C@H]1[C@H](O)[C@@H](O)C(O)O[C@@H]1CO)[C@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)CO
- InChI=1S/C12H22O11/c13-1-3-5(15)6(16)9(19)12(22-3)23-10-4(2-14)21-11(20)8(18)7(10)17/h3-20H,1-2H2/t3-,4-,5-,6+,7-,8-,9-,10-,11?,12-/m1/s1

Key: GUBGYTABKSRVRQ-PICCSMPSSA-N
InChI=1S/C12H22O11/c13-1-3-5(15)6(16)9(19)12(22-3)23-10-4(2-14)21-11(20)8(18)7(10)17/h3-20H,1-2H2/t3-,4-,5-,6+,7-,8-,9-,10-,11?,12-/m1/s1
Properties[1] Molecular formula C12H22O11 Molar mass 342.30 g/mol Appearance white powder or crystals Density 1.54 g/cm3 Melting point 160–165 °C (anhydrous)
102-103 °C (monohydrate)Solubility in water 1.080 g/mL (20 °C) Chiral rotation [α]D +140.7º (H2O, c = 10) Hazards MSDS External MSDS EU Index not listed Related compounds Related Sucrose
Lactose
Trehalose
Cellobiose (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Maltose (pronounced /ˈmɒltoʊz/), or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4)bond, formed from a condensation reaction. The isomer "isomaltose" has two glucose molecules linked through an α(1→6) bond. Maltose is the second member of an important biochemical series of glucose chains. Maltose is the disaccharide produced when amylase breaks down starch. It is found in germinating seeds such as barley as they break down their starch stores to use for food. It is also produced when glucose is caramelized. [2]
The addition of another glucose unit yields maltotriose; further additions will produce dextrins (also called maltodextrins) and eventually starch (glucose polymer).[citation needed]
Maltose can be broken down into two glucose molecules by hydrolysis. In living organisms, the enzyme maltase can achieve this very rapidly. In the laboratory, heating with a strong acid for several minutes will produce the same result. Isomaltose is broken by isomaltase.[citation needed]
The production of maltose from germinating cereals, such as barley, is an important part of the brewing process. When barley is malted, it is brought into a condition in which the concentration of maltose-producing amylases has been maximized. Mashing is the process by which these amylases convert the cereal's starches into maltose. Metabolism of maltose by yeast during fermentation then leads to the production of ethanol and carbon dioxide.[citation needed]
Maltose as food
Plain maltose has a sweet taste, about half as sweet as glucose and about one-sixth as sweet as fructose.[citation needed]
In Southern China, Taiwan, Hong Kong and Macau, maltose is a common ingredient in confectionery. The most common way to consume it is to put a layer of maltose between two pieces of biscuit (usually crackers).[citation needed]
External links
References
- ^ Weast, Robert C., ed (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-367. ISBN 0-8493-0462-8..
- ^ Journal of Food Science, July 1966.
Types of carbohydrates General: Geometry Monosaccharides Aldodiose (Glycolaldehyde)Ketopentose (Ribulose, Xylulose)
Aldopentose (Ribose, Arabinose, Xylose, Lyxose)
Deoxy sugar (Deoxyribose)>7Multiple Other oligosaccharidesGlucose/Glucan: Glycogen · Starch (Amylose, Amylopectin) · Cellulose · Dextrin/Dextran · Beta-glucan (Zymosan, Lentinan, Sizofiran) · Maltodextrin
Fructose/Fructan: Inulin · Levan beta 2→6
N-Acetylglucosamine: Chitinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Categories:- Disaccharides
- Sweeteners
- Starch
Wikimedia Foundation. 2010.



