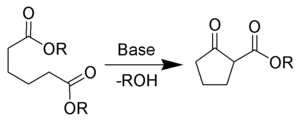
- Condensation reaction
-
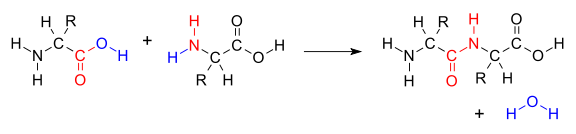 The condensation of two amino acids to form a peptide bond (red) with expulsion of water (blue)
The condensation of two amino acids to form a peptide bond (red) with expulsion of water (blue)A condensation reaction is a chemical reaction in which two molecules or moieties (functional groups) combine to form one single molecule, together with the loss of a small molecule.[1] When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride, methanol, or acetic acid. The word "condensation" suggests a process in which two or more things are brought "together" (Latin "con") to form something "dense", like in condensation from gaseous to liquid state of matter; this does not imply, however, that condensation reaction products have greater density than reactants.
When two separate molecules react, the condensation is termed intermolecular. A simple example is the condensation of two amino acids to form the peptide bond characteristic of proteins. This reaction example is the opposite of hydrolysis, which splits a chemical entity into two parts through the action of the polar water molecule, which itself splits into hydroxide and hydrogen ions.
If the union is between atoms or groups of the same molecule, the reaction is termed intramolecular condensation, and in many cases leads to ring formation. An example is the Dieckmann condensation, in which the two ester groups of a single diester molecule react with each other to lose a small alcohol molecule and form a β-ketoester product.
Contents
Mechanism
Many condensation reactions follow a nucleophilic acyl substitution or an aldol condensation reaction mechanism. Other condensations, such as the acyloin condensation are triggered by radical or single electron transfer conditions.
Condensation reactions in polymer chemistry
In one type of polymerization reaction, a series of condensation steps take place whereby monomers or monomer chains add to each other to form longer chains. This is termed 'condensation polymerization' or 'step-growth polymerization', and occurs for example in the synthesis of polyesters or nylons. It may be either a homopolymerization of a single monomer A-B with two different end groups which condense, or a copolymerization of two co-monomers A-A and B-B. Small molecules are usually liberated in these condensation steps, in contrast to polyaddition reactions with no liberation of small molecules.
In general, condensation polymers form more slowly than addition polymers, often requiring heat. They are generally lower in molecular weight. Monomers are consumed early in the reaction; the terminal functional groups remain active throughout and short chains combine to form longer chains. A high conversion rate is required to achieve high molecular weights as per Carothers' equation.
Bifunctional monomers lead to linear chains (and therefore thermoplastic polymers), but when the monomer functionality exceeds two, the product is a branched chain which may yield a thermoset polymer.
Applications
This type of reaction is used as a basis for the making of many important polymers for example: nylon, polyester and other condensation polymers and various epoxies. It is also the basis for the laboratory formation of silicates and polyphosphates. The reactions that form acid anhydrides from their constituent acids are typically condensation reactions.
Many biological transformations are condensation reactions. Polypeptide synthesis, polyketide synthesis, terpene syntheses, phosphorylation, and glycosylations are a few examples of this reaction.
A large number of such reactions are used in synthetic organic chemistry. Other examples include:
- Acyloin condensation
- Aldol condensation
- Benzoin condensation (this is not technically a condensation, but is called so for historical reasons)[1]
- Claisen condensation
- Claisen-Schmidt condensation
- Darzens condensation (glycidic ester condensation)
- Dieckmann condensation
- Guareschi-Thorpe condensation
- Knoevenagel condensation
- Michael condensation
- Pechmann condensation
- Rap-Stoermer condensation
- Self-condensation or symmetrical aldol condensation
- Ziegler condensation
See named reactions
See also
- Anabolism
- Hydrolysis
- Condensed tannins
References
- ^ a b IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1994) "Condensation Reaction".
Categories:- Condensation reactions
- Organic reactions
- Chemical reactions
Wikimedia Foundation. 2010.