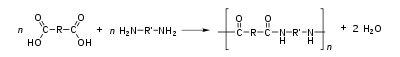- Condensation polymer
-
Condensation polymers are any kind of polymers formed through a condensation reaction, releasing small molecules as by-products such as water or methanol, as opposed to addition polymers which involve the reaction of unsaturated monomers. Types of condensation polymers include polyamides, polyacetals and polyesters.
Condensation polymerization, a form of step-growth polymerization, is a process by which two molecules join together, resulting loss of small molecules which is often water. The type of end product resulting from a condensation polymerization is dependent on the number of functional end groups of the monomer which can react.
Monomers with only one reactive group terminate a growing chain, and thus give end products with a lower molecular weight. Linear polymers are created using monomers with two reactive end groups and monomers with more than two end groups give three dimensional polymers which are crosslinked.
Dehydration synthesis often involves joining monomers with an -OH (hydroxyl) group and a freely ionized -H on either end (such as a hydrogen from the -NH2 in nylon or proteins). Normally, two or more different monomers are used in the reaction. The bonds between the hydroxyl group, the hydrogen atom and their respective atoms break forming water from the hydroxyl and hydrogen, and the polymer.
Polyester is created through ester linkages between monomers, which involve the functional groups carboxyl and hydroxyl (an organic acid and an alcohol monomer).
Nylon is another common condensation polymer. It can be manufactured by reacting di-amines with carboxyl derivatives. In this example the derivative is a di-carboxylic acid, but di-acyl chlorides are also used. Another approach used is the reaction of di-functional monomers, with one amine and one carboxylic acid group on the same molecule:
The carboxylic acids and amines link to form peptide bonds, also known as amide groups. Proteins are condensation polymers made from amino acid monomers. Carbohydrates are also condensation polymers made from sugar monomers such as glucose and galactose.
Condensation polymerization is occasionally used to form simple hydrocarbons. This method, however, is expensive and inefficient, so the addition polymer of ethene (polyethylene) is generally used.
Condensation polymers, unlike addition polymers, may be biodegradable. The peptide or ester bonds between monomers can be hydrolysed by acid catalysts or bacterial enzymes breaking the polymer chain into smaller pieces.
The most commonly known condensation polymers are proteins, fabrics such as nylon, silk, or polyester.
See also
Categories:- Polymer chemistry
- Polymerization reactions
Wikimedia Foundation. 2010.