- Dextrin
-
Dextrin 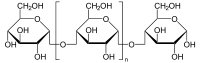
Identifiers CAS number 9004-53-9 UNII 2NX48Z0A9G 
KEGG C00721 
Properties Molecular formula (C6H10O5)n Molar mass variable Appearance white or yellow powder  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch[1] or glycogen.[2] Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds.
Dextrins can be produced from starch using enzymes like amylases, as during digestion in the human body and during malting and mashing,[3] or by applying dry heat under acidic conditions (pyrolysis or roasting). The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavour, colour, and crispness. Dextrins produced by heat are also known as pyrodextrins. During roasting under acid condition the starch hydrolyses and short chained starch parts partially rebranche with α-(1,6) bonds to the degraded starch molecule.[4]
Dextrins are white, yellow, or brown powders that are partially or fully water-soluble, yielding optically active solutions of low viscosity. Most can be detected with iodine solution, giving a red coloration; one distinguishes erythrodextrin (dextrin that colours red) and achrodextrin (giving no colour).
White and yellow dextrins from starch roasted with little or no acid is called British gum.
Contents
Uses
Yellow dextrins are used as water-soluble glues [5] in remoistable envelope adhesives and paper tubes, in the mining industry as additives in froth flotation, in the foundry industry as green strength additives in sand casting, as printing thickener for batik resist dyeing, and as binders in gouache paint.
White dextrins are used as:
- a crispness enhancer for food processing, in food batters, coatings, and glazes, (E number 1400)
- a textile finishing and coating agent to increase weight and stiffness of textile fabrics
- a thickening and binding agent in pharmaceuticals and paper coatings.
As pyrotechnic binder and fuel, they are added to fireworks and sparklers, allowing them to solidify as pellets or "stars."
Due to the rebranching, dextrins are less digestible; indigestible dextrin are developed as soluble fiber supplements for food products.
Other dextrin types
- Maltodextrin
Maltodextrin is a shortchain starch sugar, gelatin hybrid base, (dextrin) used as a food additive. It is produced also by enzymatic hydrolysis from gelatinated starch and is usually found as a creamy-white hygroscopic spraydried powder. Maltodextrin is easily digestible, being absorbed as rapidly as glucose, and might either be moderately sweet or have hardly any flavor at all.
- Cyclodextrin
The cyclical dextrins are known as cyclodextrins. They are formed by enzymatic degradation of starch by certain bacteria, for example, Bacillus macerans. Cyclodextrins have toroidal structures formed by 6-8 glucose residues.
- Amylodextrin is a linear dextrin or short chained amylose (DP 20-30) that can be produced by enzymatic hydrolysis of the alpha-1,6 glycosidic bonds or debranching amylopectin. Amylodextrin colors blue with iodine.
- (Beta) Limit dextrin is the remaining polymer produced by enzymatic hydrolysis of amylopectine with beta amylase, which cannot hydrolyse the alpha-1,6 bonds at branch points.
- (Alpha) Limit dextrin is a short chained branched amylopectine remain, produced by hydrolysis of amylopectine with alpha amylase.
- Highly branched cyclic dextrin is a dextrin produced from enzymatic breaking pf the amylopectin in clusters and using branching enzyme to form large cyclic chains.[6]
See also
Notes and references
- ^ An Introduction to the chemistry of plants - Vol II: Metabolic processes, P. Haas and T. G. Hill, London (Longmans, Green & Co.), 1913; pages 123-127
- ^ Salway, JG. Medical Biochemistry at a Glance. Second Edition. Malden, MA (Blackwell Publishing), 2006; page 66
- ^ Michael Lewis, Tom W. Young (2002), "Brewing", Kluwer Academic, ISBN 0-306-47274-0.
- ^ Alistair M. Stephen, Glyn O. Phillips, Peter A. Williams (2006), "Food polysaccharides and their applications 2nd edition", p 92-99, CRC Press, Taylor & Francis Group, ISBN 0-8247-5922-2
- ^ Jack Augustus Radley (1976). "Industrial uses of starch and its derivatives", Applied Science Publishers Ltd, ISBN 0-85334-691-7.
- ^ T. Hiroki, K. Iwao, T. Noboru,S. Yuji, Y. Mikio, Journal: Seibutsu Kogakkaishi, Vol:84; No:2; Page: 61-66 (2006), Industrial Production of Branching Enzyme and Its Application to Production of Highly Branched Cyclic Dextrin (Cluster Dextrin)[1]
External links
Types of carbohydrates General: Geometry Monosaccharides Aldodiose (Glycolaldehyde)Ketopentose (Ribulose, Xylulose)
Aldopentose (Ribose, Arabinose, Xylose, Lyxose)
Deoxy sugar (Deoxyribose)>7Multiple Other oligosaccharidesGlucose/Glucan: Glycogen · Starch (Amylose, Amylopectin) · Cellulose · Dextrin/Dextran · Beta-glucan (Zymosan, Lentinan, Sizofiran) · Maltodextrin
Fructose/Fructan: Inulin · Levan beta 2→6
N-Acetylglucosamine: Chitinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Polysaccharides
- Starch
- Food additives
- Edible thickening agents
- Pyrotechnic chemicals
Wikimedia Foundation. 2010.

