- Degree of polymerization
-
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.[1][2][3]
For a homopolymer, there is only one type of monomeric unit and the number-average degree of polymerization is given by
For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired.
Some authors, however, define DP as the number of repeat units, where for copolymers the repeat unit may not be identical to the monomeric unit.[4][5] For example, in nylon-6,6, the repeat unit contains the two monomeric units —NH(CH2)6NH— and —OC(CH2)4CO— , so that a chain of 1000 monomeric units corresponds to 500 repeat units. The degree of polymerization or chain length is then 1000 by the first (IUPAC) definition, but 500 by the second.
In polycondensation, in order to achieve a high degree of polymerization (and hence molecular weight), Xn, a high fractional monomer conversion, p, is required, as per Carothers' equation: Xn = 1/(1−p). A monomer conversion of p = 99% would be required to achieve Xn = 100.
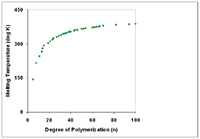
Correlation with physical properties
Polymers with identical composition but different total molecular weights may exhibit different physical properties. In general, increasing degree of polymerization correlates with higher melting temperature [6] and higher mechanical strength.
See also
References
- ^ IUPAC Definition in Compendium of Chemical Terminology (IUPAC Gold Book)
- ^ Cowie J.M.G. "Polymers: Chemistry and Physics of Modern Materials" (2nd edn Blackie 1991), p.10
- ^ Allcock H.R., Lampe F.W. and Mark J.P. "Contemporary Polymer Chemistry" (3d edn Pearson Prentice-Hall 2003), p.316
- ^ Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p.27
- ^ Rudin A. "Elements of Polymer Science and Engineering" (Academic Press 1982), p.7
- ^ Flory, P.J. and Vrij, A. J. Am. Chem. Soc.; 1963; 85(22) pp3548-3553
Categories:- Polymer chemistry
- Chemistry stubs
Wikimedia Foundation. 2010.