- P-glycoprotein
-
ATP-binding cassette, sub-family B (MDR/TAP), member 1 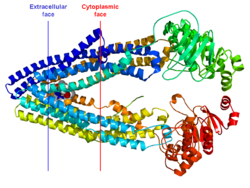
Crystallographic structure of the mouse MDR3 protein. The approximate positioning of the protein in the cell membrane is indicated by the blue (extracellular face) and red (cytoplasmic face) lines. The protein is depicted as a rainbow colored cartoon (N-terminus = blue, C-terminus = red). A cyclic peptide inhibitor QZ59 is represented by a space-filling model.[1]Available structures PDB 3G5U, 3G60, 3G61 Identifiers Symbols ABCB1; ABC20; CD243; CLCS; GP170; MDR1; MGC163296; P-gp; PGY1 External IDs OMIM: 171050 MGI: 97570 HomoloGene: 55496 GeneCards: ABCB1 Gene Gene Ontology Molecular function • nucleotide binding
• transporter activity
• ATP binding
• xenobiotic-transporting ATPase activity
• hydrolase activity
• ATPase activityCellular component • membrane fraction
• cell surface
• membrane
• integral to membraneBiological process • transport
• response to drugSources: Amigo / QuickGO RNA expression pattern 
More reference expression data Orthologs Species Human Mouse Entrez 5243 18671 Ensembl ENSG00000085563 ENSMUSG00000040584 UniProt P08183 Q9QX25 RefSeq (mRNA) NM_000927 NM_011076 RefSeq (protein) NP_000918 NP_035206 Location (UCSC) Chr 7:
86.97 – 87.18 MbChr 5:
8.67 – 8.75 MbPubMed search [1] [2] ABCB1 is differentially expressed in 97 experiments [93 up/106 dn]: 26 organism parts: kidney [2 up/0 dn], bone marrow [0 up/2 dn], ...; 29 disease states: normal [10 up/3 dn], glioblastoma [0 up/2 dn], ...; 30 cell types, 22 cell lines, 11 compound treatments and 16 other conditions. Factor Value Factor Up/Down Legend:  - number of studies the gene is up/down in
- number of studies the gene is up/down inNormal Disease state 10/3 None Compound treatment 3/0 Stromal cell Cell type 1/2 Kidney Cell type 2/0 MDA-MB-231 Cell line 0/2 Glioblastoma Disease state 0/2 Epithelial cell Cell type 0/2 HeLa Cell line 0/2 Primary Disease staging 2/0 Bone marrow Organism part 0/2 ABCB1 expression data in ATLAS P-glycoprotein 1 (permeability glycoprotein, abbreviated as P-gp or Pgp) also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1) or cluster of differentiation 243 (CD243) is a glycoprotein that in humans is encoded by the ABCB1 gene.[2] P-gp is a well-characterized ABC-transporter (which transports a wide variety of substrates across extra- and intracellular membranes) of the MDR/TAP subfamily.[3]
Pgp is extensively distributed and expressed in the intestinal epithelium, hepatocytes, renal proximal tubular cells, adrenal gland and capillary endothelial cells comprising the blood-brain and blood-testis barrier.
Contents
Function
The membrane-associated protein encoded by this gene is a member of the superfamily of ATP-binding cassette (ABC) transporters. ABC proteins transport various molecules across extra- and intra-cellular membranes. ABC genes are divided into seven distinct subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP, GCN20, White). This protein is a member of the MDR/TAP subfamily. Members of the MDR/TAP subfamily are involved in multidrug resistance. The protein encoded by this gene is an ATP-dependent drug efflux pump for xenobiotic compounds with broad substrate specificity. It is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. This protein also functions as a transporter in the blood-brain barrier.[4]
ABCB1 is an ATP-dependent efflux pump with broad substrate specificity. It likely evolved as a defense mechanism against harmful substances.
ABCB1 transports various substrates across the cell membrane including:
- Drugs such as colchicine, tacrolimus and quinidine
- Chemotherapeutic agents such as etoposide, doxorubicin, and vinblastine
- Lipids
- Steroids
- Xenobiotics
- Peptides
- Bilirubin
- Cardiac glycosides like digoxin
- Immunosuppressive agents
- Glucocorticoids like dexamethasone
- HIV-type 1 antiretroviral therapy agents like protease inhibitors and nonnucleoside reverse transcriptase inhibitors.
Its ability to transport the above substrates accounts for the many roles of ABCB1 including:
- Regulating the distribution and bioavailability of drugs
- Increased intestinal expression of P-glycoprotein can reduce the absorption of drugs that are substrates for P-glycoprotein. Thus, there is a reduced bioavailability, and therapeutic plasma concentrations are not attained. On the other hand, supratherapeutic plasma concentrations and drug toxicity may result because of decreased P-glycoprotein expression
- Active cellular transport of antineoplastics resulting in multidrug resistance to these drugs
- The removal of toxic metabolites and xenobiotics from cells into urine, bile, and the intestinal lumen
- The transport of compounds out of the brain across the blood-brain barrier
- Digoxin uptake
- Prevention of ivermectin entry into the central nervous system
- The migration of dendritic cells
- Protection of hematopoietic stem cells from toxins.[3]
Structure
Pgp is a 170 kDa transmembrane glycoprotein, which includes 10-15 kDa of N-terminal glycosylation. The N-term half of the molecule contains 6 transmembrane domains, followed by a large cytoplasmic domain with an ATP-binding site, and then a second section with 6 transmembrane domains and an ATP-binding site that shows over 65% of amino acid similarity with the first half of the polypeptide.[5] In 2009, the first structure of a mammalian P-glycoprotein was solved (3G5U).[6] The structure was derived from the mouse MDR3 gene product heterologously expressed in Pichia pastoris yeast. The structure of mouse P-gp is similar to structures of the bacterial ABC transporter MsbA (3B5W and 3B5X)Ward A, Reyes CL, Yu J, Roth CB, Chang G (November 2007). "Flexibility in the ABC transporter MsbA: Alternating access with a twist". Proc. Natl. Acad. Sci. U.S.A. 104 (48): 19005–10. doi:10.1073/pnas.0709388104. PMC 2141898. PMID 18024585. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2141898. that adopt an inward facing conformation that is believed to be important for binding substrate along the inner leaflet of the membrane. Additional structures (3G60 and 3G61) of P-gp were also solved revealing the binding site(s) of two different cyclic peptide substrate/inhibitors. The promiscuous binding pocket of P-gp is lined with aromatic amino acid side chains.
Mechanism of action
Binding of a substrate and ATP molecule occur simulatenously. Following binding of each, ATP hydrolysis shifts the substrate into a position to be excreted from the cell. Release of the phosphate (from the original ATP molecule) occurs concurrently with substrate excretion. ADP is released, and a new molecule of ATP binds to the secondary ATP-binding site. Hydrolysis and release of ADP and a phosphate molecule resets the protein.
Tissue distribution
P-glycoprotein is expressed primarily in certain cell types in the liver, pancreas, kidney, colon, and jejunum.[7]
Detecting the activity of the transporter
The activity of the transporter can be determined by both membrane ATPase and cellular calcein assays.
The ABCB1 Transporter is also used to differentiate Transitional B-cells from Naive B-cells. Dyes such as Rhodamine123 and MitoTracker Dyes from Invitrogen can be used to make this differentiation.Wirths S, Lanzavecchia A (December 2005). "ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells". Eur. J. Immunol. 35 (12): 3433–41. doi:10.1002/eji.200535364. PMID 16259010.
History
ABCB1 was first cloned and characterized using its ability to confer a multidrug resistance phenotype to cancer cells that had developed resistance to chemotherapy drugs.[3][8]
Radioactive verapamil can be used for measuring P-glycoprotein function with positron emission tomography.[9]
See also
- MDR1 defect
References
- ^ PDB 3G60; Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G (March 2009). "Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding". Science 323 (5922): 1718–22. doi:10.1126/science.1168750. PMC 2720052. PMID 19325113. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2720052.
- ^ Ueda K, Clark DP, Chen CJ, Roninson IB, Gottesman MM, Pastan I (January 1987). "The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation". J. Biol. Chem. 262 (2): 505–8. PMID 3027054. http://www.jbc.org/cgi/content/abstract/262/2/505.
- ^ a b c Dean, Michael (2002-11-01). "The Human ATP-Binding Cassette (ABC) Transporter Superfamily". National Library of Medicine (US), NCBI. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mono_001.chapter.137. Retrieved 2008-03-02.
- ^ "Entrez Gene: ABCB1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5243.
- ^ Franck Viguié (1998-03-01). "ABCB1". Atlas of Genetics and Cytogenetics in Oncology and Haematology. http://atlasgeneticsoncology.org//Genes/PGY1ID105.html. Retrieved 2008-03-02.
- ^ Stephen Aller; Jodie Yu, Andrew Ward, Yue Weng, Srinivas Chittaboina, Rupeng Zhuo, Patina M. Harrell, Yenphuong T. Trinh,Qinghai Zhang, Ina L. Urbatsch, Geoffrey Chang (2009-03-27). "Structure of P-glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding". Science (Science) 323 (5922): 1718–1722. doi:10.1126/science.1168750. PMC 2720052. PMID 19325113. http://www.sciencemag.org/cgi/content/abstract/323/5922/1718. Retrieved 2009-04-12.
- ^ Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC (November 1987). "Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues". Proc. Natl. Acad. Sci. U.S.A. 84 (21): 7735–8. doi:10.1073/pnas.84.21.7735. PMC 299375. PMID 2444983. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=299375.
- ^ Juliano RL, Ling V (1976). "A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants". Biochim. Biophys. Acta 455 (1): 152–62. doi:10.1016/0005-2736(76)90160-7. PMID 990323.
- ^ Luurtsema G, Windhorst AD, Mooijer MPJ, Herscheid A, Lammertsma AA, Franssen EJF (2002). "Fully automated high yield synthesis of (R)- and (S)-[C-11]verapamil for measuring P-glycoprotein function with positron emission tomography". Journal of Labelled Compounds & Radiopharmaceuticals 45 (14): 1199–1207. doi:10.1002/jlcr.632.
Further reading
- Ling V (1997). "Multidrug resistance: molecular mechanisms and clinical relevance". Cancer Chemother. Pharmacol. 40 Suppl (7): S3–8. doi:10.1007/s002800051053. PMID 9272126.
- Kerb R, Hoffmeyer S, Brinkmann U (2001). "ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2". Pharmacogenomics 2 (1): 51–64. doi:10.1517/14622416.2.1.51. PMID 11258197.
- Akiyama S (2002). "[Mechanisms of drug resistance and reversal of the resistance]". Hum. Cell 14 (4): 257–60. PMID 11925925.
- Brinkmann U (2002). "Functional polymorphisms of the human multidrug resistance (MDR1) gene: correlation with P glycoprotein expression and activity in vivo". Novartis Found. Symp.. Novartis Foundation Symposia 243: 207–10; discussion 210–2, 231–5. doi:10.1002/0470846356.ch15. ISBN 9780470846353. PMID 11990778.
- Váradi A, Szakács G, Bakos E, Sarkadi B (2002). "P glycoprotein and the mechanism of multidrug resistance". Novartis Found. Symp.. Novartis Foundation Symposia 243: 54–65; discussion 65–8, 180–5. doi:10.1002/0470846356.ch5. ISBN 9780470846353. PMID 11990782.
- Hegedus T, Orfi L, Seprodi A, et al. (2002). "Interaction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1". Biochim. Biophys. Acta 1587 (2–3): 318–25. PMID 12084474.
- Pallis M, Turzanski J, Higashi Y, Russell N (2003). "P-glycoprotein in acute myeloid leukaemia: therapeutic implications of its association with both a multidrug-resistant and an apoptosis-resistant phenotype". Leuk. Lymphoma 43 (6): 1221–8. doi:10.1080/10428190290026277. PMID 12152989.
- Schaich M, Illmer T (2003). "Mdr1 gene expression and mutations in Ras proto-oncogenes in acute myeloid leukemia". Leuk. Lymphoma 43 (7): 1345–54. doi:10.1080/10428190290033279. PMID 12389613.
- Fromm MF (2003). "The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans". Adv. Drug Deliv. Rev. 54 (10): 1295–310. doi:10.1016/S0169-409X(02)00064-9. PMID 12406646.
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM (2003). "P-glycoprotein: from genomics to mechanism". Oncogene 22 (47): 7468–85. doi:10.1038/sj.onc.1206948. PMID 14576852.
- Jamroziak K, Robak T (2004). "Pharmacogenomics of MDR1/ABCB1 gene: the influence on risk and clinical outcome of haematological malignancies". Hematology 9 (2): 91–105. doi:10.1080/10245330310001638974. PMID 15203864.
- Ishikawa T, Onishi Y, Hirano H, et al. (2005). "Pharmacogenomics of drug transporters: a new approach to functional analysis of the genetic polymorphisms of ABCB1 (P-glycoprotein/MDR1)". Biol. Pharm. Bull. 27 (7): 939–48. doi:10.1248/bpb.27.939. PMID 15256718.
- Lee W, Lockhart AC, Kim RB, Rothenberg ML (2005). "Cancer pharmacogenomics: powerful tools in cancer chemotherapy and drug development". Oncologist 10 (2): 104–11. doi:10.1634/theoncologist.10-2-104. PMID 15709212.
- Gambrelle J, Labialle S, Dayan G, et al. (2005). "[Multidrug resistance in uveal melanoma.]". Journal français d'ophtalmologie 28 (6): 652–9. PMID 16141933.
- Al-Shawi MK, Omote H (2006). "The Remarkable Transport Mechanism of P-glycoprotein; a Multidrug Transporter". J. Bioenerg. Biomembr. 37 (6): 489–96. doi:10.1007/s10863-005-9497-5. PMC 1459968. PMID 16691488. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1459968.
- Orlowski S, Martin S, Escargueil A (2006). "P-glycoprotein and 'lipid rafts': some ambiguous mutual relationships (floating on them, building them or meeting them by chance?)". Cell. Mol. Life Sci. 63 (9): 1038–59. doi:10.1007/s00018-005-5554-9. PMID 16721513.
- Annese V, Valvano MR, Palmieri O, et al. (2006). "Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis". World J. Gastroenterol. 12 (23): 3636–44. PMID 16773678.
- Sekine I, Minna JD, Nishio K, et al. (2007). "A literature review of molecular markers predictive of clinical response to cytotoxic chemotherapy in patients with lung cancer". Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 1 (1): 31–7. PMID 17409824.
- Kumar YS, Adukondalu D, Sathish D, Vishnu YV, Ramesh G, Latha AB, Reddy PC, Sarangapani M, Rao YM (2010). "P-Glycoprotein- and cytochrome P-450-mediated herbal drug interactions". Drug Metabol Drug Interact 25 (1-4): 3–16. doi:10.1515/DMDI.2010.006. PMID 21417789.
External links
- MeSH P-Glycoprotein
- Jessica R Oesterheld (2002-05-01). "P-glycoprotein". Mental Health Connections, Inc. Archived from the original on 2008-02-07. http://web.archive.org/web/20080207233027/http://www.mhc.com/PGP/PgpMain.HTML. Retrieved 2008-03-02.
- P-glycoprotein substrate prediction
- NextBio.com
- PharmGKB.org
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
A B C D E F G see also ABC transporter disorders
B memb: cead, trns (1A, 1C, 1F, 2A, 3A1, 3A2-3, 3D), othrCategories:- Human proteins
- ABC transporters
- Clusters of differentiation
Wikimedia Foundation. 2010.
