- ITGAV
-
Integrin alpha-V is a protein that in humans is encoded by the ITGAV gene.[1]
ITAGV encodes integrin alpha chain V. Integrins are heterodimeric integral membrane proteins composed of an alpha chain and a beta chain. Alpha V undergoes post-translational cleavage to yield disulfide-linked heavy and light chains, that combine with multiple integrin beta chains to form different integrins. Among the known associating beta chains (beta chains 1,3,5,6, and 8; 'ITGB1', 'ITGB3', 'ITGB5', 'ITGB6', and 'ITGB8'), each can interact with extracellular matrix ligands; the alpha V beta 3 integrin, perhaps the most studied of these, is referred to as the Vitronectin receptor (VNR). In addition to adhesion, many integrins are known to facilitate signal transduction.[2]
Contents
See also
References
- ^ Sosnoski DM, Emanuel BS, Hawkins AL, van Tuinen P, Ledbetter DH, Nussbaum RL, Kaos FT, Schwartz E, Phillips D, Bennett JS, et al. (Aug 1988). "Chromosomal localization of the genes for the vitronectin and fibronectin receptors alpha subunits and for platelet glycoproteins IIb and IIIa". J Clin Invest 81 (6): 1993–8. doi:10.1172/JCI113548. PMC 442653. PMID 2454952. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=442653.
- ^ "Entrez Gene: ITGAV integrin, alpha V (vitronectin receptor, alpha polypeptide, antigen CD51)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=3685.
Further reading
- Horton MA (1997). "The alpha v beta 3 integrin "vitronectin receptor".". Int. J. Biochem. Cell Biol. 29 (5): 721–5. doi:10.1016/S1357-2725(96)00155-0. PMID 9251239.
- Porter JC, Hogg N (1999). "Integrins take partners: cross-talk between integrins and other membrane receptors.". Trends Cell Biol. 8 (10): 390–6. doi:10.1016/S0962-8924(98)01344-0. PMID 9789327.
- Sajid M, Stouffer GA (2002). "The role of alpha(v)beta3 integrins in vascular healing.". Thromb. Haemost. 87 (2): 187–93. PMID 11858476.
- Cooper CR, Chay CH, Pienta KJ (2002). "The role of alpha(v)beta(3) in prostate cancer progression.". Neoplasia 4 (3): 191–4. doi:10.1038/sj/neo/7900224. PMC 1531692. PMID 11988838. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1531692.
- Cacciari B, Spalluto G (2005). "Non peptidic alphavbeta3 antagonists: recent developments.". Curr. Med. Chem. 12 (1): 51–70. PMID 15638730.
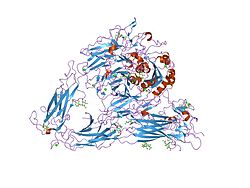
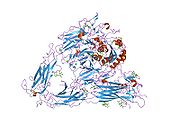
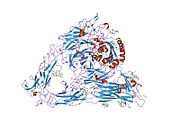
PDB gallery 1jv2: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN ALPHAVBETA31l5g: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN AVB3 IN COMPLEX WITH AN ARG-GLY-ASP LIGAND1m1x: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN ALPHA VBETA3 BOUND TO MN2+1u8c: A novel adaptation of the integrin PSI domain revealed from its crystal structureExternal links
- MeSH CD51+Antigen
- ITGAV Info with links in the Cell Migration Gateway
Alpha Beta Dimers Cytoadhesin receptor: Integrin alpha6beta4 · Glycoprotein IIb/IIIa (ITGA2B+ITGB3)
Fibrinogen receptor: Macrophage-1 antigen (CD11b+CD18)
Fibronectin receptor: Integrin alpha2beta1 · Integrin alpha4beta1 · Integrin alpha5beta1
Leukocyte-adhesion receptor: LFA-1 (CD11a+CD18) · Macrophage-1 antigen (CD11b+CD18) · Integrin alphaXbeta2 (CD11c+CD18)
Very late antigen receptor: Integrin alpha1beta1 · Integrin alpha2beta1 · Integrin alpha3beta1 · VLA-4 (CD49d+CD29) · Alpha-5 beta-1 · Integrin alpha6beta1
Vitronectin receptor: Alpha-v beta-3 · Alpha-v beta-5Categories:- Human proteins
- Membrane protein stubs
- Clusters of differentiation
Wikimedia Foundation. 2010.