- Dipeptidyl peptidase-4
-
Dipeptidyl peptidase-4 (DPP4), also known as adenosine deaminase complexing protein 2 or CD26 (cluster of differentiation 26) is a protein that, in humans, is encoded by the DPP4 gene.[1]
Contents
Function
The protein encoded by the DPP4 gene is an antigenic enzyme expressed on the surface of most cell types and is associated with immune regulation, signal transduction and apoptosis. It is an intrinsic membrane glycoprotein and a serine exopeptidase that cleaves X-proline dipeptides from the N-terminus of polypeptides.
It is a rather indiscriminate enzyme for which a diverse range of substrates are known.[2] The substrates of CD26/DPPIV are proline(or alanine)-containing peptides and include growth factors, chemokines, neuropeptides, and vasoactive peptides.
DPP4 is related to attractin, FAP, DPP8 and DPP9.
DPP4 plays a major role in glucose metabolism. It is responsible for the degradation of incretins such as GLP-1.[3]
Furthermore, it appears to work as a suppressor in the development of cancer and tumours.[4][5][6]
CD26/DPPIV plays an important role in tumor biology, and is useful as a marker for various cancers, with its levels either on the cell surface or in the serum increased in some neoplasms and decreased in others.[7]
DPP-4 also binds the enzyme adenosine deaminase specifically and with high affinity. The significance of this interaction has yet to be established.
Clinical significance
A new class of oral hypoglycemics called dipeptidyl peptidase-4 inhibitors work by inhibiting the action of this enzyme, thereby prolonging incretin effect in vivo.[8]
See also
References
- ^ Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C (July 1993). "Direct association of adenosine deaminase with a T cell activation antigen, CD26". Science 261 (5120): 466–9. doi:10.1126/science.8101391. PMID 8101391. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=8101391.
- ^ Chen X (2006). "Biochemical properties of recombinant prolyl dipeptidases DPP-IV and DPP8". Adv. Exp. Med. Biol.. Advances in Experimental Medicine and Biology 575: 27–32. doi:10.1007/0-387-32824-6_3. ISBN 978-0-387-29058-4. PMID 16700505.
- ^ Barnett A (November 2006). "DPP-4 inhibitors and their potential role in the management of type 2 diabetes". Int. J. Clin. Pract. 60 (11): 1454–70. doi:10.1111/j.1742-1241.2006.01178.x. PMID 17073841.
- ^ Pro B, Dang N (2004). "CD26/dipeptidyl peptidase IV and its role in cancer". Histol Histopathol 19 (4): 1345–51. PMID 15375776.
- ^ Masur K et al. (2006). "DPPIV inhibitors extend GLP-2 mediated tumour promoting effects on intestinal cancer cells". Regul Pept. 137 (3): 147–55. doi:10.1016/j.regpep.2006.07.003. PMID 16908079.
- ^ Wesley UV et al. (2005). "Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway". Cancer Res. 65 (4): 1325–34. doi:10.1158/0008-5472.CAN-04-1852. PMID 15735018.
- ^ Havre PA, Abe M, Urasaki Y, Ohnuma K, Morimoto C, Dang NH (2008). "The role of CD26/dipeptidyl peptidase IV in cancer". Front. Biosci. 13 (13): 1634–45. doi:10.2741/2787. PMID 17981655. http://www.bioscience.org/2008/v13/af/2787/fulltext.htm.
- ^ Rosenstock J, Zinman B (April 2007). "Dipeptidyl peptidase-4 inhibitors and the management of type 2 diabetes mellitus". Curr Opin Endocrinol Diabetes Obes 14 (2): 98–107. doi:10.1097/MED.0b013e3280a02f65. PMID 17940427.
External links
- The MEROPS online database for peptidases and their inhibitors: S09.003
- MeSH Dipeptidyl-Peptidase+IV
- Banting and Best Diabetes Centre at UT dpp4
Further reading
- Ansorge S, Bühling F, Kähne T, et al. (1997). "CD26/dipeptidyl peptidase IV in lymphocyte growth regulation". Adv. Exp. Med. Biol. 421: 127–40. PMID 9330689.
- Reinhold D, Kähne T, Steinbrecher A, et al. (2003). "The role of dipeptidyl peptidase IV (DP IV) enzymatic activity in T cell activation and autoimmunity". Biol. Chem. 383 (7–8): 1133–8. doi:10.1515/BC.2002.123. PMID 12437097.
- Sato K, Dang NH (2003). "CD26: a novel treatment target for T-cell lymphoid malignancies? (Review)". Int. J. Oncol. 22 (3): 481–97. PMID 12579300.
- de Meester I, Lambeir AM, Proost P, Scharpé S (2003). "Dipeptidyl peptidase IV substrates. An update on in vitro peptide hydrolysis by human DPPIV". Adv. Exp. Med. Biol.. Advances in Experimental Medicine and Biology 524: 3–17. doi:10.1007/0-306-47920-6_1. ISBN 0-306-47717-3. PMID 12675218.
- Koch S, Anthonsen D, Skovbjerg H, Sjöström H (2003). "On the role of dipeptidyl peptidase IV in the digestion of an immunodominant epitope in celiac disease". Adv. Exp. Med. Biol.. Advances in Experimental Medicine and Biology 524: 181–7. doi:10.1007/0-306-47920-6_22. ISBN 0-306-47717-3. PMID 12675238.
- Pro B, Dang NH (2005). "CD26/dipeptidyl peptidase IV and its role in cancer". Histol. Histopathol. 19 (4): 1345–51. PMID 15375776.
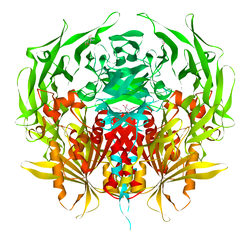
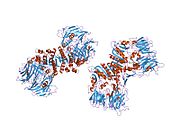
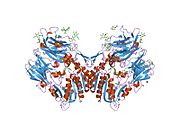
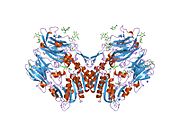
PDB gallery 1j2e: Crystal structure of Human Dipeptidyl peptidase IV1n1m: Human Dipeptidyl Peptidase IV/CD26 in complex with an inhibitor1nu6: Crystal structure of human Dipeptidyl Peptidase IV (DPP-IV)1nu8: Crystal structure of human dipeptidyl peptidase IV (DPP-IV) in complex with Diprotin A (ILI)1pfq: crystal structure of human apo dipeptidyl peptidase IV / CD261r9m: Crystal Structure of Human Dipeptidyl Peptidase IV at 2.1 Ang. Resolution.1r9n: Crystal Structure of human dipeptidyl peptidase IV in complex with a decapeptide (tNPY) at 2.3 Ang. Resolution1rwq: Human Dipeptidyl peptidase IV in complex with 5-aminomethyl-6-(2,4-dichloro-phenyl)-2-(3,5-dimethoxy-phenyl)-pyrimidin-4-ylamine1tk3: Crystal Structure Of Human Apo Dipeptidyl Peptidase IV/CD261tkr: Human Dipeptidyl Peptidase IV/CD26 inhibited with Diisopropyl FluoroPhosphate1u8e: HUMAN DIPEPTIDYL PEPTIDASE IV/CD26 MUTANT Y547F1w1i: CRYSTAL STRUCTURE OF DIPEPTIDYL PEPTIDASE IV (DPPIV OR CD26) IN COMPLEX WITH ADENOSINE DEAMINASE1wcy: Crystal Structure Of Human Dipeptidyl Peptidase IV (DPPIV) Complex With Diprotin A1x70: HUMAN DIPEPTIDYL PEPTIDASE IV IN COMPLEX WITH A BETA AMINO ACID INHIBITOR2ajl: X-ray Structure of Novel Biaryl-Based Dipeptidyl peptidase IV inhibitor2bgn: HIV-1 TAT PROTEIN DERIVED N-TERMINAL NONAPEPTIDE TRP2-TAT (1-9) BOUND TO THE ACTIVE SITE OF DIPEPTIDYL PEPTIDASE IV (CD26)2bgr: CRYSTAL STRUCTURE OF HIV-1 TAT DERIVED NONAPEPTIDES TAT(1-9) BOUND TO THE ACTIVE SITE OF DIPEPTIDYL PEPTIDASE IV (CD26)2bub: CRYSTAL STRUCTURE OF HUMAN DIPEPTIDYL PEPTIDASE IV (CD26) IN COMPLEX WITH A REVERSED AMIDE INHIBITOR2fjp: Human dipeptidyl peptidase IV/CD26 in complex with an inhibitor2g5p: Crystal structure of human dipeptidyl peptidase IV (DPPIV) complexed with cyanopyrrolidine (C5-pro-pro) inhibitor 21ac2g5t: Crystal structure of human dipeptidyl peptidase IV (DPPIV) complexed with cyanopyrrolidine (C5-pro-pro) inhibitor 21ag2g63: Crystal structure of human dipeptidyl peptidase IV (DPPIV) complexed with cyanopyrrolidine (C5-pro-pro) inhibitor 24b2hha: The structure of DPP4 in complex with an oxadiazole inhibitor2i03: Crystal structure of human dipeptidyl peptidase 4 (DPP IV) with potent alkynyl cyanopyrrolidine (ABT-279)2iit: Human dipeptidyl peptidase 4 in complex with a diazepan-2-one inhibitor2iiv: Human dipeptidyl peptidase 4 in complex with a diazepan-2-one inhibitor2ogz: Crystal structure of DPP-IV complexed with Lilly aryl ketone inhibitor2oph: Human dipeptidyl peptidase IV in complex with an alpha amino acid inhibitor2oqi: Human Dipeptidyl Peptidase IV (DPP4) with Piperidinone-constrained phenethylamine2oqv: Human Dipeptidyl Peptidase IV (DPP4) with piperidine-constrained phenethylamine2p8s: Human dipeptidyl peptidase IV/CD26 in complex with a cyclohexalamine inhibitor1-50 CD1 (a-c, 1A, 1D, 1E) · CD2 · CD3 (γ, δ, ε) · CD4 · CD5 · CD6 · CD7 · CD8 (a) · CD9 · CD10 · CD11 (a, b, c) · CD13 · CD14 · CD15 · CD16 (A, B) · CD18 · CD19 · CD20 · CD21 · CD22 · CD23 · CD24 · CD25 · CD26 · CD27 · CD28 · CD29 · CD30 · CD31 · CD32 (A, B) · CD33 · CD34 · CD35 · CD36 · CD37 · CD38 · CD39 · CD40 · CD41 · CD42 (a, b, c, d) · CD43 · CD44 · CD45 · CD46 · CD47 · CD48 · CD49 (a, b, c, d, e, f) · CD5051-100 CD51 · CD52 · CD53 · CD54 · CD55 · CD56 · CD57 · CD58 · CD59 · CD61 · CD62 (E, L, P) · CD63 · CD64 (A, B, C) · CD66 (a, b, c, d, e, f) · CD68 · CD69 · CD70 · CD71 · CD72 · CD73 · CD74 · CD78 · CD79 (a, b) · CD80 · CD81 · CD82 · CD83 · CD84 · CD85 (a, d, e, h, j, k) · CD86 · CD87 · CD88 · CD89 · CD90 · CD91- CD92 · CD93 · CD94 · CD95 · CD96 · CD97 · CD98 · CD99 · CD100101-150 CD101 · CD102 · CD103 · CD104 · CD105 · CD106 · CD107 (a, b) · CD108 · CD109 · CD110 · CD111 · CD112 · CD113 · CD114 · CD115 · CD116 · CD117 · CD118 · CD119 · CD120 (a, b) · CD121 (a, b) · CD122 · CD123 · CD124 · CD125 · CD126 · CD127 · CD129 · CD130 · CD131 · CD132 · CD133 · CD134 · CD135 · CD136 · CD137 · CD138 · CD140b · CD141 · CD142 · CD143 · CD144 · CD146 · CD147 · CD148 · CD150151-200 CD151 · CD152 · CD153 · CD154 · CD155 · CD156 (a, b, c) · CD157 · CD158 (a, d, e, i, k) · CD159 (a, c) · CD160 · CD161 · CD162 · CD163 · CD164 · CD166 · CD167 (a, b) · CD168 · CD169 · CD170 · CD171 · CD172 (a, b, g) · CD174 · CD177 · CD178 · CD179 (a, b) · CD181 · CD182 · CD183 · CD184 · CD185 · CD186 · CD191 · CD192 · CD193 · CD194 · CD195 · CD196 · CD197 · CDw198 · CDw199 · CD200201-250 CD201 · CD202b · CD204 · CD205 · CD206 · CD207 · CD208 · CD209 · CDw210 (a, b) · CD212 · CD213a (1, 2) · CD217 · CD218 (a, b) · CD220 · CD221 · CD222 · CD223 · CD224 · CD225 · CD226 · CD227 · CD228 · CD229 · CD230 · CD233 · CD234 · CD235 (a, b) · CD236 · CD238 · CD239 · CD240CE · CD240D · CD241 · CD243 · CD244 · CD246 · CD247- CD248 · CD249251-300 CD252 · CD253 · CD254 · CD256 · CD257 · CD258 · CD261 · CD262 · CD264 · CD265 · CD266 · CD267 · CD268 · CD269 · CD271 · CD272 · CD273 · CD274 · CD275 · CD276 · CD278 · CD279 · CD280 · CD281 · CD282 · CD283 · CD284 · CD286 · CD288 · CD289 · CD290 · CD292 · CDw293 · CD294 · CD295 · CD297 · CD298 · CD299301-350 Lymphoid Pre-B cell: CD10/CALLA · CD79A
mature: CD19 · CD20 · CD21/CR2 · CD23/FcεRII · CD127 · CD40
plasma cell: CD38 · CD138T/NKNK cellAllAllMyeloid CFU-GM/
MyelomonocyteCFU-MegCFU-ECD36 · CD71All (pan-myeloid)Stem cell Hydrolase: proteases (EC 3.4) 3.4.11-19: Exopeptidase Dipeptidyl peptidase (Cathepsin C, Dipeptidyl peptidase-4) · Tripeptidyl peptidase (Tripeptidyl peptidase I, Tripeptidyl peptidase II)Other/ungrouped3.4.21-24: Endopeptidase Serine proteases · Cysteine protease · Aspartic acid protease · Metalloendopeptidases
Other/ungrouped: Amyloid precursor protein secretase (Alpha secretase, Beta-secretase 1, Beta-secretase 2, Gamma secretase)3.4.99: Unknown 
This hydrolase article is a stub. You can help Wikipedia by expanding it.