- Dipeptidyl peptidase-4 inhibitor
-
Inhibitors of dipeptidyl peptidase 4, also DPP-4 inhibitors or gliptins, are a class of oral hypoglycemics that block DPP-4. They can be used to treat diabetes mellitus type 2.
The first agent of the class - sitagliptin - was approved by the FDA in 2006.[1]
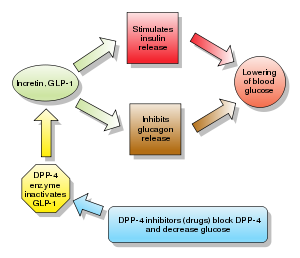
Glucagon increases blood glucose levels, and DPP-4 inhibitors reduce glucagon and blood glucose levels. The mechanism of DPP-4 inhibitors is to increase incretin levels (GLP-1 and GIP),[2][3][4] which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels.
Contents
Examples
Drugs belonging to this class are :
- sitagliptin[5] (FDA approved 2006, marketed by Merck & Co. as Januvia),
- vildagliptin[6] (EU approved 2008, marketed in the EU by Novartis as Galvus),
- saxagliptin (FDA approved in 2009, marketed as Onglyza),
- linagliptin (FDA approved in 2011, marketed as Tradjenta by Eli Lilly Co and Boehringer Ingelheim),[7]
- dutogliptin (being developed by Phenomix Corporation), Phase III[8]
- gemigliptin (being developed by LG Life Sciences,Korea)[9]
- alogliptin (developed by Takeda Pharmaceutical Company, whose FDA application for the product is currently under review)
Berberine, the common herbal dietary supplement, too inhibits dipeptidyl peptidase-4, which at least partly explains its antihyperglycemic activity.[10]
Risks and side effects
Long-term effects of DPP-4 inhibitors on mortality and morbidity are so far inconclusive, although adverse effects, including nasopharyngitis (the common cold), headache, nausea, hypersensitivity and skin reactions, have been observed in clinical studies. Consistent with this FDA approval of Novartis' DPP-4 inhibitor vildagliptin (Galvus®) was delayed because of skin lesions with blistering observed in nonhuman primate toxicology studies;[11] one year later, Novartis CEO Dan Vasella remained uncertain as to their ability to ever file to market the drug in the United States.[12] Other possible adverse effects, including hypersensitivity reactions and pancreatitis, have been reported.[13] These effects may relate to DPP-4's function in restricting the inflammatory actions of the chemokine CCL11/eotaxin, so that inhibiting DPP-4 might unleash the recruitment of inflammatory cells.[14]
Although one in vitro study found that DPP-4 inhibitors, together with GLP-2, increased proliferation and migration of colon cancer cells, which might encourage cancer cells to metastasize,[15] carcinogenicity has not been confirmed in long-term, preclinical studies of the major DPP-4 inhibitors.[citation needed]
DPP-4 appears to work as a suppressor in the development of cancer and tumours.[15][16]
See also
Further reading
- Herper, Matthew; Langreth, Robert (27 April 2006). "Diabetes Drugs to Watch". Forbes.com. http://www.forbes.com/2005/08/02/pharmaceuticals-biotechnology-diabetes-cx_mh_rl_diabetestearsheet.html. Retrieved 26 April 2009
See pages of this article for Galvus aka LAF237 (Novartis) and Januvia aka MK-0431 (Merck) - Nielsen, L (2005). "Incretin mimetics and DPP-IV inhibitors for the treatment of type 2 diabetes". Drug Discovery Today 10 (10): 703–10. doi:10.1016/S1359-6446(05)03460-4. PMID 15896683.
Includes table describing an overview of type 2 diabetes drug therapies; 76 references.
References
- ^ "FDA Approves New Treatment for Diabetes" (Press release). U.S. Food and Drug Administration. October 17, 2006. http://www.fda.gov/bbs/topics/NEWS/2006/NEW01492.html. Retrieved 2006-10-17.
- ^ McIntosh, C; Demuth, H; Pospisilik, J; Pederson, R (2005). "Dipeptidyl peptidase IV inhibitors: How do they work as new antidiabetic agents?". Regulatory Peptides 128 (2): 159–65. doi:10.1016/j.regpep.2004.06.001. PMID 15780435.
- ^ Behme, Margaret T; Dupré, John; McDonald, Thomas J (2003). "Glucagon-like peptide 1 improved glycemic control in type 1 diabetes". BMC Endocrine Disorders 3: 3. doi:10.1186/1472-6823-3-3. PMC 154101. PMID 12697069. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=154101.
- ^ Dupre, J.; Behme, M. T.; Hramiak, I. M.; McFarlane, P.; Williamson, M. P.; Zabel, P.; McDonald, T. J. (1995). "Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM". Diabetes 44 (6): 626–30. doi:10.2337/diabetes.44.6.626. PMID 7789625.
- ^ Banting and Best Diabetes Centre at UT sitagliptin
- ^ Banting and Best Diabetes Centre at UT vildagliptin
- ^ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm253501.htm
- ^ "Forest Splits With Phenomix", San Diego Business Journal, Tuesday, April 20, 2010 http://www.sdbj.com/news/2010/apr/20/forest-splits-phenomix/
- ^ http://thinklgls.com/rnd/pipeline
- ^ Al-Masri, Ihab M.; Mohammad, Mohammad K.; Tahaa, Mutasem O. (2009). "Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine". Journal of Enzyme Inhibition and Medicinal Chemistry 24 (5): 1061–6. doi:10.1080/14756360802610761. PMID 19640223.
- ^ "The FDA's Decision on Galvus". Seeking Alpha. February 28, 2007. http://seekingalpha.com/article/28241-the-fda-s-decision-on-galvus. Retrieved 2011-09-19.
- ^ Goldstein, Jacob (January 17, 2008). "Novartis Diabetes Drug May Never Be Sold in U.S.". Wall Street Journal Health Blog. Wall Street Journal. http://blogs.wsj.com/health/2008/01/17/novartis-diabetes-drug-may-never-be-sold-in-us/. Retrieved 2011-09-19. "“It’s on the cards, that we won’t refile, but it’s also on the cards that we will,” [Novartis CEO Dan Vasella] said, according to Dow Jones Newswires. “But what is certainly clear, is that refiling without new data makes no sense.""
- ^ "Dipeptidyl peptidase-4 inhibitors (‘gliptins’) for type 2 diabetes mellitus". NPS RADAR. August 1, 2010. http://www.nps.org.au/health_professionals/publications/nps_radar/2008/august_2008/gliptins. Retrieved August 6, 2010.
- ^ Forssmann, Ulf; Stoetzer, Carsten; Stephan, Michael; Kruschinski, Carsten; Skripuletz, Thomas; Schade, Jutta; Schmiedl, Andreas; Pabst, Reinhard et al. (2008). "Inhibition of CD26/Dipeptidyl Peptidase IV Enhances CCL11/Eotaxin-Mediated Recruitment of Eosinophils In Vivo". Journal of Immunology 181 (2): 1120–7. PMID 18606664. http://www.jimmunol.org/cgi/pmidlookup?view=long&pmid=18606664.
- ^ a b Masur, K; Schwartz, F; Entschladen, F; Niggemann, B; Zaenker, K (2006). "DPPIV inhibitors extend GLP-2 mediated tumour promoting effects on intestinal cancer cells". Regulatory Peptides 137 (3): 147–55. doi:10.1016/j.regpep.2006.07.003. PMID 16908079.
- ^ Wesley, U. V.; McGroarty, M; Homoyouni, A (2005). "Dipeptidyl Peptidase Inhibits Malignant Phenotype of Prostate Cancer Cells by Blocking Basic Fibroblast Growth Factor Signaling Pathway". Cancer Research 65 (4): 1325–34. doi:10.1158/0008-5472.CAN-04-1852. PMID 15735018.
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin K+ ATPMeglitinides/"glinides"GLP-1 analogsExenatide • Liraglutide • Taspoglutide† • Albiglutide† • LixisenatideDPP-4 inhibitorsAnalogs/other insulinsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsCanagliflozin† • Dapagliflozin† • Remogliflozin§ • Sergliflozin§OtherBenfluorex‡ • Tolrestat‡Pharmacology: enzyme inhibition Class Substrate Oxidoreductase (EC 1)1.1 Aldose reductase · HMG-CoA reductase
1.13 Lipoxygenase
1.17 Xanthine oxidase · Ribonucleotide reductaseTransferase (EC 2)2.1 COMT · Thymidylate synthase
2.4 PARP
2.5 Dihydropteroate synthetase · Farnesyltransferase
2.6 GABA transaminase
2.7 Nucleotidyltransferase (Integrase, Reverse transcriptase) · Protein kinase (Tyrosine-kinase (Janus kinase))Hydrolase (EC 3)3.1 Phosphodiesterase · Acetylcholinesterase · Ribonuclease
3.2 Polygalacturonase · Neuraminidase · Alpha-glucosidase
3.4 Protease: Exopeptidase (Dipeptidyl peptidase-4, ACE) · Endopeptidase (Trypsin, Renin, Matrix metalloproteinase)
3.5 Histone deacetylase · Beta-lactamaseLyase (EC 4)4.1 Dopa decarboxylase
4.2 Carbonic anhydrase
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.