- Buformin
-
Buformin 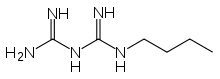
Systematic (IUPAC) name N-butylimidocarbonimidic diamide Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status Withdrawn in most countries, still available in Japan, Taiwan, and Hungary Routes Oral Pharmacokinetic data Excretion Renal Identifiers CAS number 692-13-7 
ATC code A10BA03 PubChem CID 2468 DrugBank DB04830 ChemSpider 2374 
UNII W2115E9C7B 
KEGG D00595 
ChEMBL CHEMBL39736 
Chemical data Formula C6H15N5 Mol. mass 157.217 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Buformin (1-butylbiguanide) is an oral anti-diabetic drug of the biguanide class, chemically related to metformin and phenformin. Buformin was marketed by German pharmaceutical company Grünenthal as Silubin.
Contents
Chemistry and Animal Toxicology
Buformin hydrochloride is a fine white to slightly yellow crystalline powder, odorless, with a weakly acidic bitter taste, melting point 174° to 177°, strong base, freely soluble in water, methanol and ethanol, insoluble in chloroform and ether.[1][2] Toxicity: guinea pig LD50 subcutaneous 18mg/kg; mouse LD50 intraperitoneal 140mg/kg and 300mg/kg oral[3]. Partition coefficient: log P (octanol-water) -1.20E+00; Water Solubility 7.46E+05 mg/L at 25°. Vapor pressure 1.64E-04 mm Hg at 25° (EST); Henry's Law Constant 8.14E-16 atm-m3/mole at 25° (EST). Atmospheric OH Rate Constant 1.60E-10 cm3/molecule-sec at 25°[4]
Mechanism of Action
Buformin delays absorption of glucose from the gastrointestinal tract, increases insulin sensitivity and glucose uptake into cells and inhibits synthesis of glucose by the liver. Buformin and the other biguanides are not hypoglycemic, but rather anti-hyperglycemic agents. They do not produce hypoglycemia; instead, they reduce basal and postprandial hyperglycemia in diabetics. [5]
Pharmacokinetics
After oral administration of 50 mg of buformin to volunteers, almost 90% of the applied quantity was recovered in the urine; the rate constant of elimination was found to be 0.38 hr-1. Buformin is a strong base (pKA = 11.3) and not absorbed in the stomach. After i.v. injection of about 1 mg/kg buformin-14-C, the initial serum concentration is 0.2-0.4 µg/ml. Serum level and urinary elimination rate are linearly correlated. [6] In man, after oral administration of 50 mg 14-C-buformin, the maximum serum concentration was 0.26-0.41 µg/ml. The buformin was eliminated with an average half-life of 2 h. 84% of the dose administered was found excreted unchanged in the urine. [7] Buformin is not metabolized in man.
Dosage
The daily dose of buformin is 150-300 mg by mouth. Buformin has also been available in a sustained release preparation.[8]
Side Effects
Anorexia; nausea; diarrhea; metallic taste; weight loss.
Contraindications
Diabetic coma; ketoacidosis; severe infection; trauma; other severe infections where buformin is unlikely to control the hyperglycemia; renal or hepatic impairment; heart failure; recent myocardial infarct; dehydration; alcoholism; conditions likely to predispose to lactic acidosis.
Toxicity
Buformin was withdrawn from the market in most countries due to an elevated risk of causing lactic acidosis, but is still available and prescribed in Hungary, [9][10][11][12] Taiwan, [13] and Japan. [14] The lactic acidosis occurred only in patients with a buformin plasma level of greater than 0.60 µg/ml and was rare in patients with normal renal function. [15][16][17] In one report the toxic oral dose was 329 ± 30mg/day in 24 patients who developed lactic acidosis on buformin. Another group of 24 patients on 258 ± 25mg/day did not develop lactic acidosis on buformin.[18]
Anti-Cancer Properties
Buformin, along with Phenformin and Metformin, inhibits the growth and development of cancer. [19][20][21][22] The anti-cancer property of these drugs is due to their ability to disrupt the Warburg effect and revert the cytosolic glycolysis characteristic of cancer cells to normal oxidation of pyruvate by the mitochondria.[23] Metformin reduces liver glucose production in diabetics and disrupts the Warburg effect in cancer by AMPK activation and inhibition of the mTor pathway. [24]
History
Buformin was synthesized as an oral antidiabetic in 1957.[25]
References
- ^ Jacker HJ. [New Pharmacologic Products. 2. Buformin For Oral Therapy Of Diabetes]. Pharm Prax. 1964;10:247-9.
- ^ Eustace George Coverly Clarke, Judith Berle, Pharmaceutical Society of Great Britain. Dept. of Pharmaceutical Sciences. Isolation and identification of drugs in pharmaceuticals, body fluids and post-mortem material, Volume 1. Pharmaceutical Press 1974, p226
- ^ Shroff JR, Bandurco V, Desai R, Kobrin S, Cervoni P. Chemistry and hypoglycemic activity of benzimidoylpyrazoles. J Med Chem 1981 Dec;24(12):1521-5.
- ^ United States National Library of Medicine ChemLDplus advanced database
- ^ Enrique Ravina, Hugo Kubinyi. The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. Wiley. 2011 p 215
- ^ Beckmann R. The fate of biguanides in man. Ann N Y Acad Sci. 1968 Mar 26;148(3):820-32.
- ^ Beckmann R, Lintz W, Schmidt-Böthelt E. Evaluation of a sustained release form of the oral antidiabetic butylbiguanide (Silubin retard). Eur J Clin Pharmacol. 1971 Sep;3(4):221-8.
- ^ Gustav Kuschinsky, Heinz Lüllmann. Textbook of pharmacology. Academic Press p 225, 1973
- ^ Hankó B, Tukarcs E, Kumli P, Vincze Z. Antidiabetic drug utilization in Hungary. Pharm World Sci. 2005 Jun;27(3):263-5.
- ^ Hankó BZ, Reszegi CA, Kumli P, Vincze Z. [Practice of antidiabetic therapy in Hungary]. Acta Pharm Hung. 2005;75(2):77-86.
- ^ Jerry L. Schlesser, Gale Research Inc. Drugs available abroad. Derwent Publications, Ltd - 1990 p28
- ^ Verdonck L, Sangster B, van Heijst A, de Groot G, Maes R (1981). "Buformin concentrations in a case of fatal lactic acidosis". Diabetologia 20 (1): 45–6. doi:10.1007/BF01789112. PMID 7202882.
- ^ Chou CH, Cheng CL, Huang CC. A validated HPLC method with ultraviolet detection for the determination of buformin in plasma. Biomed Chromatogr. 2004 May;18(4):254-8.
- ^ Takeda Announces Submission Of Application For Additional Indication Of Actos In Japan; Concomitant Therapy With Biguanides For Type 2 Diabetes. Medical News Today. 28 Jan 2007
- ^ Wittmann P, Haslbeck M, Bachmann W, Mehnert H. [Lactic acidosis in diabetics on biguanides (author's translation)] Deutsche Medizinische Wochenschrift 102(1):5-10, 1977
- ^ Berger W, Mehnert-Aner S, Mülly K, Heierli C, Ritz R. [10 cases of lactic acidosis during biguanide therapy (buformin and phenformin)]. Schweizerische medizinische Wochenschrift. 106:1830-1834, 1976
- ^ Deppermann D, Heidland A, Ritz E, Hörl W (1978). "[Lactic acidosis--a possible complication in buformin-treated diabetics (author's transl)]". Klin Wochenschr 56 (17): 843–53. PMID 713413.
- ^ Luft D, Schmülling RM, Eggstein M. Lactic acidosis in biguanide-treated diabetics: a review of 330 cases. Diabetologia. 1978 Feb;14(2):75-87.
- ^ Vladimir N. Anisimov. Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Experimental Gerontology Volume 38, Issue 10, October 2003, Pages 1041-1049
- ^ Valery A. Alexandrov, Vladimir N. Anisimov, Natalia M. Belous, Inna A. Vasilyeva and Vera B. Mazon. The inhibition of the transplacental blastomogenic effect of nitrosomethylurea by postnatal administration of buformin to rats. Carcinogenesis Volume 1, Issue 12 Pp. 975-978, 1980
- ^ Anisimov VN, Ostroumova MN, Dil'man VM. Inhibition of the blastomogenic effect of 7,12-dimethylbenz(a)anthracene in female rats by buformin, diphenin, a polypeptide pineal extract and L-DOPA. Bulletin of Experimental Biology and Medicine. Volume 89, Number 6, 819-822, 1980
- ^ Vladimir N. Anisimov, Lev M. Berstein, Irina G. Popovich, Mark A. Zabezhinski, Peter A. Egormin, Margarita L. Tyndyk, Ivan V. Anikin, Anna V. Semenchenko, Anatoli I. Yashin. Central and Peripheral Effects of Insulin/IGF-1 Signaling in Aging and Cancer: Antidiabetic Drugs as Geroprotectors and Anticarcinogens. Annals of the New York Academy of Sciences. 1057:220-234, 2005
- ^ Matthew G. Vander Heiden, Lewis C. Cantley, and Craig B. Thompson. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 324 (5930): 1029-1033, 2009.
- ^ Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005 Dec 9;310(5754):1642-6.
- ^ Seymour L. Shapiro et al. Salts Of N-Amylbiguanide. US Patent number: 2961377; Filing date: Aug 5, 1957; Issue date: 1960
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin Metformin# • Buformin‡ • Phenformin‡K+ ATPMeglitinides/"glinides"GLP-1 analogsExenatide • Liraglutide • Taspoglutide† • Albiglutide† • LixisenatideAnalogs/other insulinsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsCanagliflozin† • Dapagliflozin† • Remogliflozin§ • Sergliflozin§OtherBenfluorex‡ • Tolrestat‡Categories:- Guanidines
- Withdrawn drugs
- Biguanides
Wikimedia Foundation. 2010.
