- Gliclazide
-
Not to be confused with glipizide or glyburide.
Gliclazide 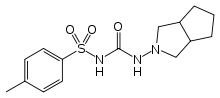
Systematic (IUPAC) name N-(hexahydrocyclopenta[c]pyrrol-2(1H)-ylcarbamoyl)-4-methylbenzenesulfonamide Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information Pregnancy cat. ? Legal status ? Pharmacokinetic data Half-life 10.4 hours Identifiers CAS number 21187-98-4 
ATC code A10BB09 PubChem CID 3475 DrugBank APRD00460 ChemSpider 3356 
UNII G4PX8C4HKV 
KEGG D01599 
ChEBI CHEBI:31654 
ChEMBL CHEMBL427216 
Chemical data Formula C15H21N3O3S Mol. mass 323.412 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Gliclazide is an oral hypoglycemic (anti-diabetic drug) and is classified as a sulfonylurea. It is marketed as Glizid, Glyloc and Reclide in India and Diamicron in Canada. In the Philippines, Servier markets it as Diamicron MR, like in most countries across the world. Many generic equivalents are also available e.g. Glubitor-OD, Clizid. It is not marketed in the United States. A modified-release formulation is also marketed. Its classification has been ambiguous, as literature uses it as both a first-generation [1] and second-generation[2] sulfonylurea. Gliclazide was proven to protect human pancreatic beta-cells from hyperglycemia-induced apoptosis.[3] It was also proven to have a potent antiatherogenic effect in type 2 diabetes.[4]
Contents
Form and composition
Each immediate-release tablet contains 80 mg. Modified release formulations contain 30 mg and 60 mg of gliclazide. In the Philippines, all three formulations are available.[citation needed]
Indication
Gliclazide is used for control of hyperglycemia in gliclazide-responsive diabetes mellitus of stable, mild, non-ketosis prone, type 2 diabetes. It is used when diabetes cannot be controlled by proper dietary management and exercise or when insulin therapy is not appropriate.[citation needed]
Mode of action
Gliclazide selectively binds to sulfonylurea receptors (SUR-1) on the surface of the pancreatic beta-cells. It was shown to provide cardiovascular protection as it does not bind to sulfonylurea receptors (SUR-2A) in the heart.[5] This binding effectively closes the K+ ion channels. This decreases the efflux of potassium from the cell which leads to the depolarization of the cell. This causes voltage dependent Ca++ ion channels to open increasing the Ca++ influx. The calcium can then bind to and activate calmodulin which in turn leads to exocystosis of insulin vesicles leading to insulin release.[citation needed]
Dosage
The dosage for the 80 mg formulation is 40 to 320 mg daily in two divided doses, while the 30 mg and 60 mg modified release formulation may be given at a dose of 30 to 120 mg once daily at breakfast.[citation needed]
Properties
- Hypoglycemic sulfonylurea, restoring first peak of insulin secretion, increasing insulin sensitivity.[citation needed]
- Glycemia-independent hemovascular effects, antioxidant effect.[citation needed]
- No active circulating metabolites.[citation needed]
Contraindications
- Type 1 diabetes[citation needed]
- Hypersensitivity to sulfonylureas[citation needed]
- Severe renal or hepatic failure[citation needed]
- Pregnancy and lactation[citation needed]
- Miconazole coprescription[citation needed]
Metabolism
Gliclazide undergoes extensive metabolism to several inactive metabolites in humans, mainly methylhydroxygliclazide and carboxygliclazide. CYP2C9 is involved in the formation of hydroxygliclazde in human liver microsomes and in a panel of recombinant human P450sin vitro.[6][7] But the pharmacokinetics of gliclazide MR are affected mainly by CYP2C19 genetic polymorphism instead of CYP2C9 genetic polymorphism.[8][9]
Interactions
Hyperglycemic action may be caused by danazol, chlorpromazine, glucocorticoids, progestogens, or β-2 agonists. Its hypoglycemic action may be potentiated by phenylbutazone, alcohol, fluconazole, β-blockers, and possibly ACE inhibitors. It has been found that rifampin increases gliclazide metabolism in humans in vivo.[10]
Adverse effects
- Hypoglycemia - while it was proven to have the same efficacy as glimepiride, one of the newer sulfonylureas, the European GUIDE study has shown that it has approximately 50% fewer confirmed hypoglycaemic episodes in comparison with glimepiride.[11]
- Gastrointestinal disturbance (reported)[citation needed]
- Skin reactions (rare)[citation needed]
- Hematological disorders (rare)[citation needed]
- Hepatic enzyme rises (exceptional)[citation needed]
Overdosage
Gliclazide overdose may cause severe hypoglycemia, requiring urgent administration of glucose by IV and monitoring.[citation needed]
References
- ^ Ballagi-Pordány, György; Köszeghy, Anna; Koltai, Mária-Zsófia; Aranyi, Zoltán; Pogátsa, Gábor (1990). "Divergent cardiac effects of the first and second generation hypoglycemic sulfonylurea compounds". Diabetes Research and Clinical Practice 8 (2): 109–14. doi:10.1016/0168-8227(90)90020-T. PMID 2106423.
- ^ Shimoyama, Tatsuhiro; Yamaguchi, Shinya; Takahashi, Kazuto; Katsuta, Hidenori; Ito, Eisuke; Seki, Hiroyuki; Ushikawa, Kenji; Katahira, Hiroshi et al. (2006). "Gliclazide protects 3T3L1 adipocytes against insulin resistance induced by hydrogen peroxide with restoration of GLUT4 translocation". Metabolism 55 (6): 722–30. doi:10.1016/j.metabol.2006.01.019. PMID 16713429.
- ^ Del Guerra, S; Grupillo, M; Masini, M; Lupi, R; Bugliani, M; Torri, S; Boggi, U; Del Chiaro, M et al. (2007). "Gliclazide protects human islet beta-cells from apoptosis induced by intermittent high glucose". Diabetes/Metabolism Research and Reviews 23 (3): 234–8. doi:10.1002/dmrr.680. PMID 16952202.
- ^ Katakami, N.; Yamasaki, Y.; Hayaishi-Okano, R.; Ohtoshi, K.; Kaneto, H.; Matsuhisa, M.; Kosugi, K.; Hori, M. (2004). "Metformin or gliclazide, rather than glibenclamide, attenuate progression of carotid intima-media thickness in subjects with type 2 diabetes". Diabetologia 47 (11): 1906–13. doi:10.1007/s00125-004-1547-8. PMID 15565373.
- ^ Lawrence, C. L.; Proks, P.; Rodrigo, G. C.; Jones, P.; Hayabuchi, Y.; Standen, N. B.; Ashcroft, F. M. (2001). "Gliclazide produces high-affinity block of K ATP channels in mouse isolated pancreatic beta cells but not rat heart or arterial smooth muscle cells". Diabetologia 44 (8): 1019–25. doi:10.1007/s001250100595. PMID 11484080.
- ^ Rieutord, A; Stupans, I; Shenfield, GM; Gross, AS (1995). "Gliclazide hydroxylation by rat liver microsomes". Xenobiotica 25 (12): 1345–54. doi:10.3109/00498259509061922. PMID 8719909.
- ^ Elliot, David J.; Lewis, Benjamin C.; Gillam, Elizabeth M. J.; Birkett, Donald J.; Gross, Annette S.; Miners, John O.; Miners, JO (2007). "Identification of the human cytochromes P450 catalysing the rate-limiting pathways of gliclazide elimination". British Journal of Clinical Pharmacology 64 (4): 450–7. doi:10.1111/j.1365-2125.2007.02943.x. PMC 2048545. PMID 17517049. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2048545.
- ^ Zhang, Yifan; Si, Dayong; Chen, Xiaoyan; Lin, Nan; Guo, Yingjie; Zhou, Hui; Zhong, Dafang (2007). "Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics of gliclazide MR in Chinese subjects". British Journal of Clinical Pharmacology 64 (1): 67–74. doi:10.1111/j.1365-2125.2007.02846.x. PMC 2000619. PMID 17298483. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2000619.
- ^ Xu, H; Williams, K M; Liauw, W S; Murray, M; Day, R O; McLachlan, A J (2009). "Effects of St John's wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide". British Journal of Pharmacology 153 (7): 1579–86. doi:10.1038/sj.bjp.0707685. PMC 2437900. PMID 18204476. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2437900.
- ^ Park, J; Kim, KA; Park, PW; Park, CW; Shin, JG (2003). "Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide". Clinical Pharmacology & Therapeutics 74 (4): 334–40. doi:10.1016/S0009-9236(03)00221-2. PMID 14534520.
- ^ Schernthaner, G.; Grimaldi, A.; Di Mario, U.; Drzewoski, J.; Kempler, P.; Kvapil, M.; Novials, A.; Rottiers, R. et al. (2004). "GUIDE study: Double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients". European Journal of Clinical Investigation 34 (8): 535–42. doi:10.1111/j.1365-2362.2004.01381.x. PMID 15305887.
External links
- Official website for Diamicron MR
- SERVIER
- Advance clinical trial on diabetes
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin K+ ATP1st generation: Acetohexamide • Carbutamide • Chlorpropamide • Tolbutamide • Tolazamide
2nd generation: Glibenclamide (Glyburide)# • Glipizide • Gliquidone • Glyclopyramide • Glimepiride • Gliclazide •Meglitinides/"glinides"GLP-1 analogsExenatide • Liraglutide • Taspoglutide† • Albiglutide† • LixisenatideAnalogs/other insulinsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsCanagliflozin† • Dapagliflozin† • Remogliflozin§ • Sergliflozin§OtherBenfluorex‡ • Tolrestat‡Categories:- Sulfonylureas
- Cyclopentapyrroles
- Servier
Wikimedia Foundation. 2010.
