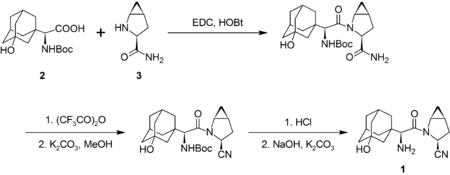- Saxagliptin
-
Saxagliptin 
Systematic (IUPAC) name (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-1-adamantyl)
acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrileClinical data AHFS/Drugs.com Consumer Drug Information MedlinePlus a610003 Licence data EMA:Link, US FDA:link Pregnancy cat. ? Legal status POM (UK) ℞-only (US) Routes Oral Identifiers CAS number 361442-04-8 
ATC code A10BH03 PubChem CID 11243969 DrugBank DB06335 ChemSpider 9419005 
UNII 8I7IO46IVQ 
ChEMBL CHEMBL385517 
Chemical data Formula C18H25N3O2 Mol. mass 315.41 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Saxagliptin (rINN), previously identified as BMS-477118, is a new oral hypoglycemic (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs.[1] Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug. A New Drug Application for saxagliptin in the treatment of type 2 diabetes was submitted to the FDA in June 2008. It was based on a drug development program with 8 randomized trials: 1 phase 2 dose-ranging (2.5–100 mg/d) study; 6 phase 3, 24-week controlled trials with additional controlled follow-up from 12 to 42 months, double-blinded throughout; and one 12-week mechanism-of-action trial with a 2-year follow-up period.[2] In June 2008, it was announced that Onglyza would be the trade name under which saxagliptin will be marketed[3].
The FDA approved Saxagliptan with brand name Onglyza on July 31, 2009[4].
Dipeptidyl peptidase-4's role in blood glucose regulation is thought to be through degradation of GIP[5] and the degradation of GLP-1.[5][6]
Bristol-Myers Squibb announced on 27 December 2006 that Otsuka Pharmaceutical Co. has been granted exclusive rights to develop and commercialize the compound in Japan. Under the licensing agreement, Otsuka will be responsible for all development costs, but Bristol-Myers Squibb retains rights to co-promote saxagliptin with Otsuka in Japan.[7] Further, on 11 January 2007 it was announced that Bristol-Myers Squibb and AstraZeneca would work together to complete development of the drug and in subsequent marketing.[8]
Contents
Production
Saxagliptin is produced industrially by Bristol-Myers Squibb by the amide coupling of N-Boc-3-hydroxyadamantylglycine (2) and methanoprolineamide (3) with EDC. The former is commercially available, whereas the latter is available as the N-Boc analog. The prolineamide moiety is subsequently dehydrated with trifluoroacetic anhydride to give the cyanide as the trifluoracetate ester, which is hydrolyzed. Removal of the Boc protecting group, followed by neutralization gives the desired product (1):[9]
How saxagliptin works
Saxagliptin is part of a class of diabetes medications called dipeptidyl peptidase-4 (DPP-4) inhibitors. DPP-4 is an enzyme that breaks down incretin hormones. As a DPP-4 inhibitor, Saxagliptin slows down the breakdown of incretin hormones, increasing the level of these hormones in the body. It is this increase in incretin hormones that is responsible for the beneficial actions of Saxagliptin, including increasing insulin production in response to meals and decreasing the amount of glucose (sugar) that the liver produces.[10]
Because incretin hormones are more active in response to higher blood sugar levels (and are less active in response to low blood sugar), the risk of dangerously low blood sugar (hypoglycemia) is low with Saxagliptin.
Efficacy
Oral saxagliptin 2.5 or 5 mg once daily suppresses DPP4 activity for 24 hours[11], significantly improving mean HbA1c levels (relative to placebo) in well designed, 24-week trials in treatment-naive patients with type 2 diabetes.[12] Combination therapy with saxagliptin 5 mg once daily and metformin was more effective than saxagliptin or metformin monotherapy.[12] When the relative benefits of increasing the dose of a sulfonylurea or adding saxagliptin were assessed in a study of 768 patients, combination treatments were shown to have a significantly greater impact on fasting blood glucose than increasing the tested glibenclamide dose alone.[11]
Safety
In 4148 patients studdied, 3 adverse reactions were seen higher in saxaglyptin vs placebo. Table 1: Adverse Reactions (Regardless of Investigator Assessment of Causality) in Placebo-Controlled Trials* Reported in ≥ 5% of Patients Treated with ONGLYZA (saxagliptin tablets) 5 mg and More Commonly than in Patients Treated with Placebo. [13]
Number (%) of Patients ONGLYZA 5 mg N=882 Placebo N=799 Upper respiratory tract infection 68 (7.7) 61 (7.6) Urinary tract infection 60 (6.8) 49 (6.1) Headache 57 (6.5) 47 (5.9) [14] - The 5 placebo-controlled trials include two monotherapy trials and one add-on combination therapy trial with each of the following: metformin, thiazolidinedione, or glyburide. Table shows 24-week data regardless of glycemic rescue. [15]
Tolerability
Both monotherapy and combination therapy with other agents was generally well tolerated in clinical trials.[12]
Cancer risk of DPP-4 inhibitors
The DPP-4 enzyme is known to be involved in the suppression of certain malignancies, particularly in limiting the tissue invasion of these tumours. Inhibiting the DPP-4 enzymes may allow some cancers to progress[16][17]. A study of DPP-4 inhibition in human non-small cell lung cancer (NSCLC) concluded that "DPPIV functions as a tumor suppressor, and its downregulation may contribute to the loss of growth control in NSCLC cells.[18]
The risk of cancer suppression with DPP-4 down-regulation applies to all the DPP-4 inhibitors on the market in addition to saxagliptin (sitagliptin and vildagliptin)
See also
- Dipeptidyl peptidase 4
- Development of dipeptidyl peptidase-4 inhibitors
References
- ^ Augeri D et al. (2005). "Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes". Journal of Medicinal Chemistry 48 (15): 5025–5037. doi:10.1021/jm050261p. PMID 16033281.
- ^ Robert Frederich, MD, PhD et al. (May 2010). "A Systematic Assessment of Cardiovascular Outcomes in the Saxagliptin Drug Development Program for Type 2 Diabetes". Postgraduate Medicine. 122 (3): 16–27. doi:10.3810/pgm.2010.05.2138. PMID 20463410. http://www.postgradmed.com/index.php?article=2138#none.
- ^ Bloomberg.com: U.S
- ^ Telegram (2 August 2009). "FDA approves diabetes drug from two area manufacturers". Worcester Telegram & Gazette Corp.. http://www.telegram.com/article/20090802/NEWS/908020328/1002. Retrieved 2009-08-02.
- ^ a b Mentlein, R; Gallwitz, B; Schmidt, WE (15 June 1993). "Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum". European Journal of Biochemistry 214 (3): 829–835. doi:10.1111/j.1432-1033.1993.tb17986.x. PMID 8100523.
- ^ Ahrén, Bo; Landin-Olsson, Mona; Jansson, Per-Anders; Svensson, Maria; Holmes, David; Schweizer, Anja (May 2004). "Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes". Journal of Clinical Endocrinology & Metabolism 89 (5): 2078–2084. doi:10.1210/jc.2003-031907. PMID 15126524. http://jcem.endojournals.org/cgi/content/full/89/5/2078. Retrieved 2007-01-11.
- ^ "Bristol-Myers Squibb and Otsuka Pharmaceutical Co., Ltd. Announce Exclusive Licensing Agreement for Diabetes Compound Saxagliptin in Japan" (Press release). Bristol-Myers Squibb. December 27, 2006. http://investor.bms.com/phoenix.zhtml?c=106664&p=irol-newsArticle&ID=944899&highlight=. Retrieved 2006-12-27.
- ^ Associated Press (11 January 2007). "AstraZeneca teams with Bristol-Myers on diabetes drugs". Delaware News-Journal. http://www.delawareonline.com/apps/pbcs.dll/article?AID=/20070111/BUSINESS/70111024/-1/NLETTER02. Retrieved 2007-01-11.[dead link]
- ^ Savage, Scott A.; Jones, Gregory S.; Kolotuchin, Sergei; Ramrattan, Shelly Ann; Vu, Truc; Waltermire, Robert E. (2009). "Preparation of Saxagliptin, a Novel DPP-IV Inhibitor". Org. Process Res. Dev. 13: 091016152805096. doi:10.1021/op900226j.
- ^ [1] Diabetes info
- ^ a b "New Drugs: Saxagliptin". Australian Prescriber (34): 89–91. June 2011. http://www.australianprescriber.com/magazine/34/3/89/91.
- ^ a b c Dhillon, S; Weber, J. (2009). "Saxagliptin". Drugs 69 (15): 2103–2114. doi:10.2165/11201170-000000000-00000. PMID 19791828. http://adisonline.com/drugs/abstract/2009/69150/Saxagliptin.5.aspx.
- ^ [2] RXlist - saxagliptin
- ^ [3] RXlist - saxagliptin
- ^ [4] RXlist - saxagliptin
- ^ Pro B, Dang NH (October 2004). "CD26/dipeptidyl peptidase IV and its role in cancer". Histol. Histopathol. 19 (4): 1345–51. PMID 15375776. http://www.hh.um.es/Abstracts/Vol_19/19_4/19_4_1345.htm.
- ^ Wesley UV, McGroarty M, Homoyouni A (February 2005). "Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway". Cancer Res. 65 (4): 1325–34. doi:10.1158/0008-5472.CAN-04-1852. PMID 15735018.
- ^ Wesley, U; et al (2004). "Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells.". Int J Cancer 109 (6): 855–866. doi:10.1002/ijc.20091. PMID 15027119. http://www.ncbi.nlm.nih.gov/pubmed/15027119.
External links
- Official site
- Banting and Best Diabetes Centre at UT dpp4
- The race to get DPP-4 inhibitors to market - Forbes.com
- Walker, Emily P.. "Saxagliptin First Diabetes Drug to Pass FDA Cardiovascular Safety Review". http://www.medpagetoday.com/Washington-Watch/Washington-Watch/13555.
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin K+ ATPMeglitinides/"glinides"GLP-1 analogsExenatide • Liraglutide • Taspoglutide† • Albiglutide† • LixisenatideAnalogs/other insulinsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsCanagliflozin† • Dapagliflozin† • Remogliflozin§ • Sergliflozin§OtherBenfluorex‡ • Tolrestat‡
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.