- Pioglitazone
-
Pioglitazone 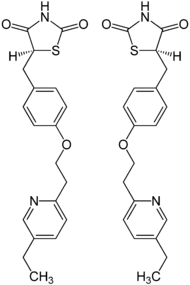
Systematic (IUPAC) name (RS)-5-(4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl)thiazolidine-2,4-dione Clinical data Trade names Actos AHFS/Drugs.com monograph MedlinePlus a699016 Licence data EMA:Link, US FDA:link Pregnancy cat. C Legal status POM (UK) ℞-only (US) Routes oral Pharmacokinetic data Protein binding >99% Metabolism liver (CYP2C8) Half-life 3–7 hours Excretion in bile Identifiers CAS number 111025-46-8 
ATC code A10BG03 PubChem CID 4829 DrugBank APRD00653 ChemSpider 4663 
UNII X4OV71U42S 
KEGG D08378 
ChEBI CHEBI:8228 
ChEMBL CHEMBL595 
Chemical data Formula C19H20N2O3S Mol. mass 356.44 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pioglitazone is a prescription drug of the class thiazolidinedione (TZD) with hypoglycemic (antihyperglycemic, antidiabetic) action. Pioglitazone is marketed as trademarks Actos in the USA, Canada, the UK and Germany, Glustin in Europe,"Glizone" and "Pioz" in India by Zydus CND and USV respectively and Zactos in Mexico by Takeda Pharmaceuticals. Actos was the tenth-best selling drug in the U.S. in 2008, with sales exceeding $2.4 billion.[1] Its cardiovascular safety profile compares favorably with rosiglitazone (Avandia), which was withdrawn after concerns about an increased risk of cardiac events, but pioglitazone has subsequently been found to be associated with bladder tumors and has been withdrawn in some countries.
Contents
Pharmacology
Pioglitazone selectively stimulates the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) and to a lesser extent PPAR-α.[2][3] It modulates the transcription of the insulin-sensitive genes involved in the control of glucose and lipid metabolism in the muscle, adipose tissue, and the liver. As a result, pioglitazone reduces insulin resistance in the liver and peripheral tissues; increases the expense of insulin-dependent glucose; decreases withdrawal of glucose from the liver; reduces quantity of glucose, insulin and glycated hemoglobin in the bloodstream. Although not clinically significant, pioglitazone decreases the level of triglycerides and increases that of high-density lipoproteins (HDL) without changing low-density lipoproteins (LDL) and total cholesterol in patients with disorders of lipid metabolism, although statins are the drug of choice for this.
More recently,[when?] pioglitazone and other active TZDs have been shown to bind to the outer mitochondrial membrane protein mitoNEET with affinity comparable to that of pioglitazone for PPARγ.[4][5]
Indications and usage
Pioglitazone is used for the treatment of diabetes mellitus type 2 (previously known as non-insulin-dependent diabetes mellitus, NIDDM) in monotherapy and in combination with a sulfonylurea, metformin, or insulin. Pioglitazone has also been used to treat non-alcoholic steatohepatitis (fatty liver), but this use is presently considered experimental.[6] Pioglitazone has also been found to reduce the risk of conversion from prediabetes to diabetes mellitus type 2 by 72%. [7]
Contraindication
Pioglitazone cannot be used in patients with a known hypersensitivity to pioglitazone, other thiazolidinediones or any of components of its pharmaceutical forms. It is ineffective and possibly harmful in diabetes mellitus type 1 and diabetic ketoacidosis. Its safety in pregnancy, lactation (breastfeeding) and people under 18 is not established.
Given previous experiences with the related drug troglitazone, acute diseases of the liver are regarded as a contraindication for pioglitazone.
Pioglitazone and all other drugs of its class (Thiazolidinediones) are absolutely contraindicated in patients with heart failure.
Side effects
A press release by GlaxoSmithKline in February 2007 noted that there is a greater incidence of fractures of the upper arms, hands and feet in female diabetics given rosiglitazone compared with those given metformin or glyburide. The information was based on data from the ADOPT trial. Following release of this statement, Takeda also admitted that pioglitazone has similar implications for female patients.[citation needed]
The risk of hypoglycemia is low in the absence of other drugs that lower blood glucose.
Pioglitazone can cause fluid retention and peripheral edema. As a result, it may precipitate congestive heart failure (which worsens with fluid overload in those at risk). It may cause anemia. Mild weight gain is common due to increase in subcutaneous adipose tissue. In studies, patients on pioglitazone had an increased proportion of upper respiratory tract infection, sinusitis, headache, myalgia and tooth problems.
On July 30, 2007 an Advisory Committee of the Food and Drug Administration concluded that the use of rosiglitazone for the treatment of type 2 diabetes was associated with a greater risk of "myocardial ischemic events" when compared to placebo, but when compared to other diabetes drugs, there was no increased risk. Pioglitazone is currently being reviewed. A meta-analysis released subsequently showed that pioglitazone reduced the number of ischemic cardiac events rather than increase the risk, but increases CHF.[8] The PERISCOPE study compared pioglitazone with glimepiride in diabetics; atherosclerotic plaque volume was measured and followed over time. Glimepiride therapy had highly significant progression of plaque volume over time of 0.73 percent. In comparison, pioglitazone had a -0.16 percent regression in plaque volume. This is the first study to show that diabetic therapy slowed progression of atherosclerosis. Therapy with pioglitazone raised HDL, and lowered triglyceride and hsCRP; these are all beneficial effects on risk factors for coronary artery disease, however to date, no oral anti-diabetic drug has been shown to reduce the risk of cardiovascular complications.[9] Chronic administration of the drug has led to occasional instances of cholestatic hepatitis, reversible upon drug discontinuation.[10]
On June 9, 2011 the French Agency for the Safety of Health Products decided to withdraw pioglitazone in regards to high risk of bladder cancer [11] On June 10, 2011 Germany's Federal Institute for Drugs and Medical Devices also advised doctors not to prescribe the medication until further investigation of the cancer risk had been conducted.[12]
On June 15, 2011 the U.S. FDA announced that pioglitazone use for more than one year may be associated with an increased risk of bladder cancer, and that the information about this risk will be added to the Warnings and Precautions section of the label for pioglitazone-containing medicines. The patient Medication Guide for these medicines will also be revised to include information on the risk of bladder cancer.[13]
Drug interactions
Combination with sulfonylureas or insulin reciprocally exponentiate risk of hypoglycemia. Therapy with pioglitazone increased risk for pregnancy in those taking oral contraception.
Formulations
Pioglitazone as Actos is supplied in oral tablets containing 15, 30 or 45 mg of pioglitazone base. It is also available in combination with metformin as ActoplusMet (tablets containing 15 mg pioglitazone and either 500 or 850 mg of metformin), Competact (tablets containing 15 mg pioglitazone and 850 mg of metformin) or in combination with glimepiride as Duetact (tablets containing 30 mg pioglitazone and either 2 or 4 mg of glimepiride).
References
- ^ Details for Actos.
- ^ Gillies, PS; Dunn, CJ (August 2000). "Pioglitazone". Drugs 60 (2): 333–43; discussion 344–5. doi:10.2165/00003495-200060020-00009. PMID 10983737.
- ^ Smith U (September 2001). "Pioglitazone: mechanism of action". Int J Clin Pract Suppl (121): 13–8. PMID 11594239.
- ^ Colca, JR; McDonald, WG; Waldon, DJ; Leone, JW; Lull, JM; Bannow, CA; Lund, ET; Mathews, WR (February 2004). "Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe". Am. J. Physiol. Endocrinol. Metab. 286 (2): E252–60. doi:10.1152/ajpendo.00424.2003. PMID 14570702. http://ajpendo.physiology.org/content/286/2/E252.full.pdf+html.
- ^ Paddock, ML; Wiley, SE; Axelrod, HL; Cohen, AE; Roy, M; Abresch, EC; Capraro, D; Murphy, AN et al. (September 2007). "MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone". Proc. Natl. Acad. Sci. U.S.A. 104 (36): 14342–7. doi:10.1073/pnas.0707189104. PMC 1963346. PMID 17766440. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1963346.
- ^ Belfort, R; Harrison, SA; Brown, K; Darland, C; Finch, J; Hardies, J; Balas, B; Gastaldelli, A et al. (November 2006). "A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis". N. Engl. J. Med. 355 (22): 2297–307. doi:10.1056/NEJMoa060326. PMID 17135584.
- ^ DeFronzo, Ralph A.. "Pioglitazone for Diabetes Prevention in Impaired Glucose Tolerance". N Eng J Med 2011; 364: 1104-1115. http://www.nejm.org/doi/full/10.1056/NEJMoa1010949. Retrieved March 29, 2011.
- ^ Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (2007). "Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials". JAMA 298 (10): 1180–8. doi:10.1001/jama.298.10.1180. PMID 17848652.
- ^ Nissen SE, Nicholls SJ, Wolski K (2008). "Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes". JAMA 299 (13): 1561. doi:10.1001/jama.299.13.1561. PMID 18378631. http://jama.ama-assn.org/cgi/content/full/299.13.1561v1.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1271-1272.
- ^ http://www.lefigaro.fr/flash-actu/2011/06/09/97001-20110609FILWWW00505-info-le-figaro-lantidiabetique-actos-retire-du-marche.php.
- ^ Topham, James (June 10, 2011). "UPDATE 2-Germany joins France in suspending top Takeda drug". Reuters. http://www.reuters.com/article/2011/06/10/takeda-germany-idUSL3E7HA0X920110610.
- ^ http://www.fda.gov/Drugs/DrugSafety/ucm259150.htm | FDA Drug Safety Communication: Update to ongoing safety review of Actos (pioglitazone) and increased risk of bladder cancer|.
External links
- Official site
- Pioglitazone FAQ
- U.S. National Library of Medicine: Drug Information Portal - Pioglitazonee
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin K+ ATPMeglitinides/"glinides"GLP-1 analogsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsOtherBenfluorex‡ • Tolrestat‡Categories:- Eli Lilly and Company
- Phenol ethers
- Pyridines
- Thiazolidinediones
Wikimedia Foundation. 2010.
