- Glibenclamide
-
Glibenclamide 

Systematic (IUPAC) name 5-chloro-N-(4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenethyl)-2-methoxybenzamide Clinical data AHFS/Drugs.com International Drug Names MedlinePlus a684058 Licence data US FDA:link Pregnancy cat. C(AU) B(US) Legal status POM (UK) ℞-only (US) Routes Oral Pharmacokinetic data Protein binding Extensive Metabolism Hepatic hydroxylation (CYP2C9-mediated) Half-life 10 hours Excretion Renal and biliary Identifiers CAS number 10238-21-8 
ATC code A10BB01 PubChem CID 3488 IUPHAR ligand 2414 DrugBank APRD00233 ChemSpider 3368 
UNII SX6K58TVWC 
KEGG D00336 
ChEBI CHEBI:5441 
ChEMBL CHEMBL472 
Chemical data Formula C23H28ClN3O5S Mol. mass 494.004 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Glibenclamide (INN), also known as glyburide (USAN), is an antidiabetic drug in a class of medications known as sulfonylureas, closely related to sulfa drugs. It was developed in 1966 in a cooperative study between Boehringer Mannheim (now part of Roche) and Hoechst (now part of Sanofi-Aventis).[1]
It is sold in doses of 1.25 mg, 2.5 mg and 5 mg, under the trade names Diabeta, Glynase and Micronase in the United States and Daonil, Semi-Daonil and Euglucon in the United Kingdom and Delmide in India.
It is also sold in combination with metformin under the trade names Glucovance and Glibomet.
Contents
Uses
It is used in the treatment of type II diabetes. As of 2007[update], it is one of only two oral antidiabetics in the World Health Organization Model List of Essential Medicines (the other being metformin).[2] As of 2003, in the United States, it was the most popular sulfonylurea.[3]
Additionally, recent research shows that glyburide improves outcome in animal stroke models by preventing brain swelling. A retrospective study showed that in type 2 diabetic patients already taking glyburide, there was improved NIH stroke scale scores on discharge compared to diabetic patients not taking glyburide.[citation needed]
Mechanism of action
The drug works by inhibiting ATP-sensitive potassium channels[4] in pancreatic beta cells. This inhibition causes cell membrane depolarization opening voltage-dependent calcium channel. This results in an increase in intracellular calcium in the beta cell and subsequent stimulation of insulin release.
Side effects and contraindications
This drug is a major cause of drug induced hypoglycemia. Cholestatic jaundice is noted.
Recently published data suggest glibenclamide is associated with significantly higher annual mortality when combined with metformin than other insulin-secreting medications, after correcting for other potentially confounding patient characteristics. The safety of this combination has been questioned.[5] Gilbenclamide causes cholestasis as the major side effect.
Glibenclamide has been demonstrated to block the protection offered by myocardial preconditioning in dogs.[6]
Synthesis
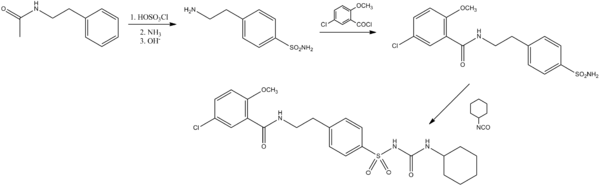
The N-acetyl derivative of β-phenethylamine is reacted with chlorosulfonic acid to form the para sulfonyl chloride derivative. This is then subjected to ammonolysis, followed by base-catalyzed removal of the acetamide. This is then acylated with 2-methoxy-5-chlorobenzoic acid chloride to give the amide intermediate. This is then reacted with cyclohexyl isocyanate to yield the sulfonylurea glibenclamide.
Hsi, R. S. P. (1973). "Synthesis of carbon-14 and tritium labeled glyburide". Journal of Labelled Compounds 9: 91. doi:10.1002/jlcr.2590090112.
References
- ^ Transdermal composition containing glibenclamide and benzyl alcohol. 3 May 2003.
- ^ (March 2007) WHO Model List of Essential MedicinesPDF (612 KiB), 15th edition, World Health Organization, p. 21. Retrieved on 2007-11-19.
- ^ Riddle MC (February 2003). "Editorial: sulfonylureas differ in effects on ischemic preconditioning--is it time to retire glyburide?". J. Clin. Endocrinol. Metab. 88 (2): 528–30. doi:10.1210/jc.2002-021971. PMID 12574174. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=12574174.
- ^ Serrano-Martín X, Payares G, Mendoza-León A (December 2006). "Glibenclamide, a Blocker of K+ATP Channels, Shows Antileishmanial Activity in Experimental Murine Cutaneous Leishmaniasis". Antimicrob. Agents Chemother. 50 (12): 4214–6. doi:10.1128/AAC.00617-06. PMC 1693980. PMID 17015627. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=17015627.
- ^ Monami M, Luzzi C, Lamanna C, Chiasserini V, Addante F, Desideri CM, Masotti G, Marchionni N, Mannucci E (2006). "Three-year mortality in diabetic patients treated with different combinations of insulin secretagogues and metformin". Diabetes Metab Res Rev 22 (6): 477–82. doi:10.1002/dmrr.642. PMID 16634115.
- ^ { author = Gross GJ and Auchampach JA | title = Blockade of ATP -sensitive potassium channels prevents myocardial preconditioning in dogs | journal = Circ Res | volume = 84| pages = 973–979 | year = 1992 }
Oral anti-diabetic drugs and Insulin analogs (A10) Insulin K+ ATP1st generation: Acetohexamide • Carbutamide • Chlorpropamide • Tolbutamide • Tolazamide
2nd generation: Glibenclamide (Glyburide)# • Glipizide • Gliquidone • Glyclopyramide • Glimepiride • Gliclazide •Meglitinides/"glinides"GLP-1 analogsfast-acting (Insulin lispro • Insulin aspart • Insulin glulisine) • short-acting (Regular insulin) • long-acting (Insulin glargine • Insulin detemir • NPH insulin) • ultra-long-acting (Insulin degludec†) • inhalable Exubera‡Other Amylin analogSGLT2 inhibitorsOtherBenfluorex‡ • Tolrestat‡
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.